Variations in Circulating Tumor Microenvironment-Associated Proteins in Non-Muscle Invasive Bladder Cancer Induced by Mitomycin C Treatment
- PMID: 40806542
- PMCID: PMC12347783
- DOI: 10.3390/ijms26157413
Variations in Circulating Tumor Microenvironment-Associated Proteins in Non-Muscle Invasive Bladder Cancer Induced by Mitomycin C Treatment
Abstract
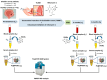
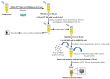
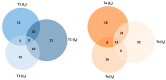
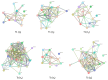
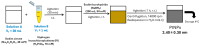
Mitomycin C (MMC) is a widely employed chemotherapeutic agent, particularly in non-muscle invasive bladder cancer (NMIBC), where it functions by inducing DNA cross-linking and promoting tumor cell apoptosis. However, the tumor microenvironment (TME) significantly influences the therapeutic efficacy of MMC. Among the key regulators within the TME, the complement system and the coagulation pathway play a crucial role in modulating immune responses to cancer therapies, including MMC. This article explores the interaction between platinum nanoparticles (PtNPs) with human serum (HS) of NMIBC patients (T1 and Ta subtypes) at three different points: before the chemotherapy instillation of MMC (t0) and three (t3) and six months (t6) after the treatment with MMC. This novel nanoproteomic strategy allowed the identification of a TME proteomic signature associated with the response to MMC treatment. Importantly, two proteins involved in the immune response were found to be deregulated across all patients (T1 and Ta subtypes) during MMC treatment: prothrombin (F2) downregulated and complement component C7 (C7) upregulated. By understanding how these biomarker proteins interact with MMC treatment, novel therapeutic strategies can be developed to enhance treatment outcomes and overcome resistance in NMIBC.
Keywords: SWATH-MS; complement system; mitomycin C (MMC); non-muscle invasive bladder cancer (NMIBC); platinum nanoparticles (PtNPs); protein corona (PC); tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A Systematic Review and Meta-analysis of Chemoablation for Non-muscle-invasive Bladder Cancer.Eur Urol Focus. 2023 May;9(3):463-479. doi: 10.1016/j.euf.2022.12.003. Epub 2022 Dec 12. Eur Urol Focus. 2023. PMID: 36517409
-
Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer.Cochrane Database Syst Rev. 2003;(3):CD003231. doi: 10.1002/14651858.CD003231. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2015 Nov 07;(11):CD003231. doi: 10.1002/14651858.CD003231.pub2. PMID: 12917955 Updated.
-
Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review.BJU Int. 2012 Feb;109(4):496-505. doi: 10.1111/j.1464-410X.2011.10880.x. BJU Int. 2012. PMID: 22313502
-
A systematic review of the efficacy of intravesical electromotive drug administration therapy for non-muscle invasive bladder cancer.Urol Oncol. 2023 Apr;41(4):166-176. doi: 10.1016/j.urolonc.2022.09.016. Epub 2022 Oct 31. Urol Oncol. 2023. PMID: 36328923
-
Intravesical electromotive drug administration for non-muscle invasive bladder cancer.Cochrane Database Syst Rev. 2017 Sep 12;9(9):CD011864. doi: 10.1002/14651858.CD011864.pub2. Cochrane Database Syst Rev. 2017. PMID: 28898400 Free PMC article.
References
-
- Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Dominguez Escrig J.L., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ) Eur. Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. - DOI - PubMed
-
- Scilipoti P., Ślusarczyk A., Angelis M., Soria F., Pradere B., Krajewski W., D’Andrea D., Mari A., Giudice F., Pichler R., et al. European Association of Urology Young Academic Urologists Urothelial Carcinoma Working Group. The Role of Mitomycin C in Intermediate-risk Non-muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2024;7:1293–1302. doi: 10.1016/j.euo.2024.06.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

