Fused-Linked and Spiro-Linked N-Containing Heterocycles
- PMID: 40806564
- PMCID: PMC12347284
- DOI: 10.3390/ijms26157435
Fused-Linked and Spiro-Linked N-Containing Heterocycles
Abstract
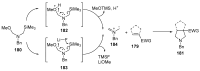
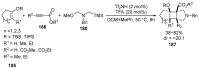
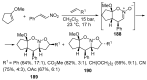
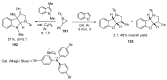
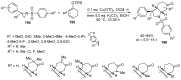
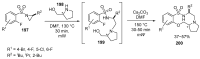
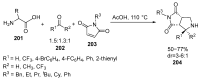
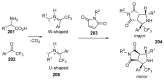
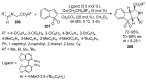
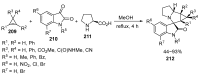
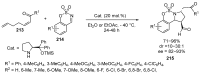
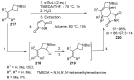
Fused and spiro nitrogen-containing heterocycles play an important role as structural motifs in numerous biologically active natural products and pharmaceuticals. The review summarizes various approaches to the synthesis of three-, four-, five-, and six-membered fused and spiro heterocycles with one or two nitrogen atoms. The assembling of the titled compounds via cycloaddition, oxidative cyclization, intramolecular ring closure, and insertion of sextet intermediates-carbenes and nitrenes-is examined on a vast number of examples. Many of the reactions proceed with high regio-, stereo-, or diastereoselectivity and in excellent, up to quantitative, yield, which is of principal importance for the synthesis of chiral drug-like compounds. For most unusual and hardly predictable transformations, the mechanisms are given or referred to.
Keywords: aziridines and azetidines; fused heterocycles; imidazolines; pyrazolines; pyrrolidines; spiro heterocycles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
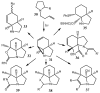
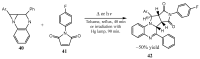
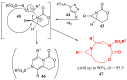
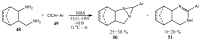






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Reactivity of Anomalous Aziridines for Versatile Access to High Fsp3 Amine Chemical Space.Acc Chem Res. 2025 Jan 21;58(2):231-249. doi: 10.1021/acs.accounts.4c00670. Epub 2024 Dec 30. Acc Chem Res. 2025. PMID: 39834235 Free PMC article.
-
Analysis of the structural diversity of heterocycles amongst European medicines agency approved pharmaceuticals (2014-2023).RSC Med Chem. 2025 Aug 11. doi: 10.1039/d5md00403a. Online ahead of print. RSC Med Chem. 2025. PMID: 40837083 Free PMC article. Review.
-
Convergence of Competitive Azidation, Elimination vs Substitution, and Preferential Ring Formation: Metal-Free, One-Pot Route to Oxa-Ring Fused Triazoles from Functionalized Polyols.ACS Omega. 2025 Jul 8;10(28):31128-31137. doi: 10.1021/acsomega.5c05041. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727762 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
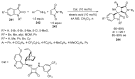
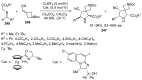
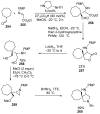
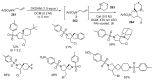
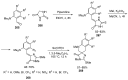
- Barreca M., Spanò V., Rocca R., Bivacqua R., Gualtieri G., Raimondi M.V., Gaudio E., Bortolozzi R., Manfreda L., Bai R., et al. Identification of pyrrolo [3,4:3,4] cyclohepta [1,2-d][1,2] oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023;254:115372. doi: 10.1016/j.ejmech.2023.115372. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

