Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways
- PMID: 40806567
- PMCID: PMC12347301
- DOI: 10.3390/ijms26157434
Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways
Abstract
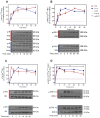
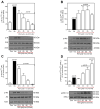
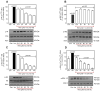
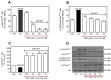
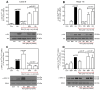
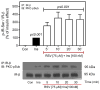
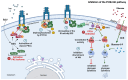
Resveratrol (RSV), a polyphenol found in a variety of berries and wines, is known for its anti-inflammatory, anticancer, and antioxidant properties. It has been suggested that RSV may play a role in the regulation of metabolic disorders, including diabetes and insulin resistance. However, in recent years, it has been reported to completely inhibit Akt kinase function in liver cells. Akt is a central protein involved in the metabolic function of insulin and is regulated by the phosphatidylinositol-3-kinase (PI3K) pathway. In this study, we examined the effect of RSV on insulin-induced insulin receptor (IR) phosphorylation and proteins involved in the PI3K/Akt pathway in a hepatic cell model, clone 9 (C9), and in hepatoma cells, Hepa 1-6 (H1-6). In both cell lines, RSV inhibited tyrosine phosphorylation of IR and insulin-induced activation of Akt. We also evaluated the effect of RSV on the activation of protein tyrosine phosphatase 1B (PTP1B), which is associated with IR dephosphorylation, and found that RSV increased PTP1B-Tyr152 phosphorylation in a time- and concentration-dependent manner. Furthermore, we found that the protein kinase C (PKC) inhibitors BIM and Gö6976 prevented the inhibition of Akt phosphorylation by RSV and increased the phosphorylation of Ser/Thr residues in IR, suggesting that PKC is involved in the inhibition of the insulin pathway by RSV. Thus, classical PKC isoforms impair the PI3K/Akt pathway at the IR and GSK3 and GS downstream levels; however, IRS-Tyr632 phosphorylation remains unaffected. These results suggest that RSV can lead to insulin resistance by activating PTP1B and PKC, consequently affecting glucose homeostasis in hepatic cells.
Keywords: hepatic cells; insulin receptor; insulin resistance; protein kinase C; protein tyrosine phosphatase; resveratrol.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Developmental switch from prolonged insulin action to increased insulin sensitivity in protein tyrosine phosphatase 1B-deficient hepatocytes.Endocrinology. 2007 Feb;148(2):594-608. doi: 10.1210/en.2006-0644. Epub 2006 Oct 26. Endocrinology. 2007. PMID: 17068137
-
Resveratrol aggravated H2O2-induced the HK-2 cell damage by inhibiting AKT phosphorylation.PLoS One. 2025 Jul 31;20(7):e0327135. doi: 10.1371/journal.pone.0327135. eCollection 2025. PLoS One. 2025. PMID: 40743123 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

