Physicochemical and Computational Study of the Encapsulation of Resv-4'-LA and Resv-4'-DHA Lipophenols by Natural and HP-β-CDs
- PMID: 40806583
- PMCID: PMC12347287
- DOI: 10.3390/ijms26157454
Physicochemical and Computational Study of the Encapsulation of Resv-4'-LA and Resv-4'-DHA Lipophenols by Natural and HP-β-CDs
Abstract
This study investigates the self-assembly and host-guest complexation behaviour of novel resveratrol-based lipophenols (LipoResv)-resveratrol-4'-linoleate (Resv-4'-LA) and resveratrol-4'-docosahexaenoate (Resv-4'-DHA)-with hydroxypropyl-β-cyclodextrins (HP-β-CDs). These amphiphilic molecules display surfactant-like properties, forming micellar aggregates in aqueous media. Fluorescence spectroscopy was used to determine the critical micelle concentration (CMC), revealing that LipoResv exhibit significantly lower CMC values than their free fatty acids, indicating higher hydrophobicity. The formation of inclusion complexes with HP-β-CDs was evaluated based on changes in CMC values and further confirmed by dynamic light scattering (DLS) and molecular modelling analyses. Resv-4'-LA formed 1:1 complexes (Kc = 720 M-1), while Resv-4'-DHA demonstrated a 1:2 stoichiometry with lower affinity constants (K1 = 17 M-1, K2 = 0.18 M-1). Environmental parameters (pH, temperature, and ionic strength) significantly modulated CMC and binding constants. Computational docking and molecular dynamics simulations supported the experimental findings by revealing the key structural determinants of the host-guest affinity and micelle stabilization. Ligand efficiency (LE) analysis further aligned with the experimental data, favouring the unmodified fatty acids. These results highlight the versatile encapsulation capacity of HP-β-CDs for bioactive amphiphile molecules and support their potential applications in drug delivery and functional food systems.
Keywords: bioactive compounds; cyclodextrins; fatty acids; lipophenols; micelles; nanoencapsulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
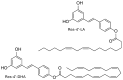

 LA,
LA,  0.25 mM HP-β-CDs/LA; (B)
0.25 mM HP-β-CDs/LA; (B)  DHA,
DHA,  0.25 mM HP-β-CDs/DHA; (C)
0.25 mM HP-β-CDs/DHA; (C)  Resv-4′-LA,
Resv-4′-LA,  0.25 mM HP-β-CDs/Resv-4′-LA; and (D)
0.25 mM HP-β-CDs/Resv-4′-LA; and (D)  Resv-4′-DHA,
Resv-4′-DHA,  0.25 mM HP-β-CDs/Resv-4′-DHA.
0.25 mM HP-β-CDs/Resv-4′-DHA.
 0 mM HP-β-CDs;
0 mM HP-β-CDs;  1 mM HP-β-CDs;
1 mM HP-β-CDs;  2 mM HP-β-CDs;
2 mM HP-β-CDs;  5 mM HP-β-CDs; and
5 mM HP-β-CDs; and  10 mM HP-β-CDs.
10 mM HP-β-CDs.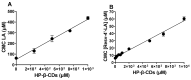

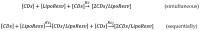
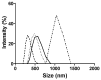
 ), Resv-4′-LA 50 µM (
), Resv-4′-LA 50 µM ( ), Resv-4′-DHA 10 µM (
), Resv-4′-DHA 10 µM ( ), and Resv-4′-DHA 50 µM (
), and Resv-4′-DHA 50 µM ( ) in PBS at 35 °C and pH 7.0.
) in PBS at 35 °C and pH 7.0.
 ), Resv-4′-LA 50 µM with 10 mM HP-β-CDs (
), Resv-4′-LA 50 µM with 10 mM HP-β-CDs ( ) in PBS, Resv-4′-LA 50 µM (
) in PBS, Resv-4′-LA 50 µM ( ), and Resv-4′-LA 50 µM with 10 mM HP-β-CDs (
), and Resv-4′-LA 50 µM with 10 mM HP-β-CDs ( ) in MilliQ water at 35 °C and pH 7.0.
) in MilliQ water at 35 °C and pH 7.0.
References
-
- Crauste C., Vercauteren J., Veas F., Durand T., Blondeau N. Uses of Lipophenolic Compounds. No. 11,419,841. U.S. Patent. 2022 August 23;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

