Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism
- PMID: 40806594
- PMCID: PMC12347391
- DOI: 10.3390/ijms26157467
Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism
Abstract
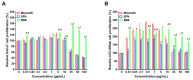
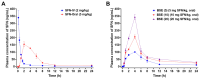
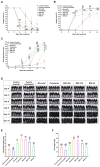
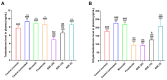
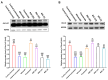
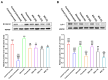
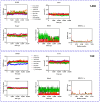
Androgenetic alopecia (AGA) is a common progressive hair loss disorder driven by elevated dihydrotestosterone (DHT) levels, leading to follicular miniaturization. This study investigated sulforaphane-rich broccoli sprout extract (BSE) as a potential oral therapy for AGA. BSE exhibited dose-dependent proliferative and migratory effects on keratinocytes, dermal fibroblasts, and dermal papilla cells, showing greater in vitro activity than sulforaphane (SFN) and minoxidil under the tested conditions, while maintaining low cytotoxicity. In a testosterone-induced AGA mouse model, oral BSE significantly accelerated hair regrowth, with 20 mg/kg achieving 99% recovery by day 15, alongside increased follicle length, density, and hair weight. Mechanistically, BSE upregulated hepatic and dermal DHT-metabolizing enzymes (Akr1c21, Dhrs9) and activated Wnt/β-catenin signaling in the skin, suggesting dual actions via androgen metabolism modulation and follicular regeneration. Pharmacokinetic analysis revealed prolonged SFN plasma exposure following BSE administration, and in silico docking showed strong binding affinities of key BSE constituents to Akr1c2 and β-catenin. No systemic toxicity was observed in liver histology. These findings indicate that BSE may serve as a safe, effective, and multitargeted natural therapy for AGA. Further clinical studies are needed to validate its efficacy in human populations.
Keywords: androgenetic alopecia; broccoli sprout extract; dihydrotestosterone; hair regeneration; hepatic DHT metabolism; sulforaphane.
Conflict of interest statement
Suk-Jung Oh was employed by the Research & Development, Ecoworld Pharm Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

