Loss of SVIP Results in Metabolic Reprograming and Increased Retention of Very-Low-Density Lipoproteins in Hepatocytes
- PMID: 40806595
- PMCID: PMC12347863
- DOI: 10.3390/ijms26157465
Loss of SVIP Results in Metabolic Reprograming and Increased Retention of Very-Low-Density Lipoproteins in Hepatocytes
Abstract
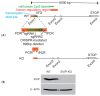
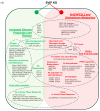
Perturbations in the tightly regulated processes of VLDL biosynthesis and secretion can directly impact both liver and cardiovascular health. Patients with metabolic disorders have an increased risk of developing hepatic steatosis, which can lead to cirrhosis. These associated metabolic risks underscore the importance of discerning the role of different cellular proteins involved in VLDL biogenesis, transport, and secretion. Small VCP-Interacting Protein (SVIP) has been identified as a component of VLDL transport vesicles and VLDL secretion. This study evaluates the cellular effects stemming from the CRISPR-Cas9-mediated depletion of SVIP in rat hepatocytes. The SVIP-knockout (KO) cells display an increased VLDL retention with elevated intracellular levels of ApoB100 and neutral lipid staining. RNA sequencing studies reveal an impaired PPARα and Nrf2 signaling in the SVIP KO cells, implying a state of metabolic reprograming, with a shift from fatty acid uptake, synthesis, and oxidation to cells favoring the activation of glucose by impaired glycogen storage and increased glucose release. Additionally, SVIP KO cells exhibit a transcriptional profile indicative of acute phase response (APR) in hepatocytes. Many inflammatory markers and genes associated with APR are upregulated in the SVIP KO hepatocytes. In accordance with an APR-like response, the cells also demonstrate an increase in mRNA expression of genes associated with protein synthesis. Together, our data demonstrate that SVIP is critical in maintaining hepatic lipid homeostasis and metabolic balance by regulating key pathways such as PPARα, Nrf2, and APR.
Keywords: PPARs; SVIP; VLDL secretion; dyslipidemia; lipids; triglyceride.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Fenofibrate Treatment Inhibits Very-Low-Density Lipoprotein Transport Vesicle Formation by Reducing Sar1b Protein Expression.Int J Mol Sci. 2025 May 15;26(10):4720. doi: 10.3390/ijms26104720. Int J Mol Sci. 2025. PMID: 40429862 Free PMC article.
-
Establishing the Role of Liver Fatty Acid-Binding Protein in Post-Golgi Very-Low-Density Lipoprotein Trafficking Using a Novel Fluorescence-Based Assay.Int J Mol Sci. 2025 Mar 7;26(6):2399. doi: 10.3390/ijms26062399. Int J Mol Sci. 2025. PMID: 40141042 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Effect of Inflammation and Infection on Lipids and Lipoproteins.2025 Jun 18. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2025 Jun 18. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 26561701 Free Books & Documents. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

