Genome-Editing Tools for Lactic Acid Bacteria: Past Achievements, Current Platforms, and Future Directions
- PMID: 40806610
- PMCID: PMC12347395
- DOI: 10.3390/ijms26157483
Genome-Editing Tools for Lactic Acid Bacteria: Past Achievements, Current Platforms, and Future Directions
Abstract
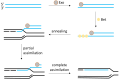
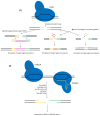
Lactic acid bacteria (LAB) are central to food, feed, and health biotechnology, yet their genomes have long resisted rapid, precise manipulation. This review charts the evolution of LAB genome-editing strategies from labor-intensive RecA-dependent double-crossovers to state-of-the-art CRISPR and CRISPR-associated transposase systems. Native homologous recombination, transposon mutagenesis, and phage-derived recombineering opened the door to targeted gene disruption, but low efficiencies and marker footprints limited throughput. Recent phage RecT/RecE-mediated recombineering and CRISPR/Cas counter-selection now enable scar-less point edits, seamless deletions, and multi-kilobase insertions at efficiencies approaching model organisms. Endogenous Cas9 systems, dCas-based CRISPR interference, and CRISPR-guided transposases further extend the toolbox, allowing multiplex knockouts, precise single-base mutations, conditional knockdowns, and payloads up to 10 kb. The remaining hurdles include strain-specific barriers, reliance on selection markers for large edits, and the limited host-range of recombinases. Nevertheless, convergence of phage enzymes, CRISPR counter-selection and high-throughput oligo recombineering is rapidly transforming LAB into versatile chassis for cell-factory and therapeutic applications.
Keywords: CRISPR-transposase; CRISPR/Cas9; Cre/lox; food biotechnology; genome editing; homologous recombination; lactic acid bacteria; probiotic engineering; recombineering; transposon mutagenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Phage-based delivery of CRISPR-associated transposases for targeted bacterial editing.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2504853122. doi: 10.1073/pnas.2504853122. Epub 2025 Jul 25. Proc Natl Acad Sci U S A. 2025. PMID: 40711918 Free PMC article.
-
CRISPR-Cas systems of lactic acid bacteria and applications in food science.Biotechnol Adv. 2024 Mar-Apr;71:108323. doi: 10.1016/j.biotechadv.2024.108323. Epub 2024 Feb 10. Biotechnol Adv. 2024. PMID: 38346597 Review.
-
Efficient genome engineering in Agrobacterium tumefaciens C58 using recombineering assisted by CRISPR/Cas9.J Biotechnol. 2025 Oct;406:99-104. doi: 10.1016/j.jbiotec.2025.07.005. Epub 2025 Jul 4. J Biotechnol. 2025. PMID: 40619069
-
A recombineering-based platform for high-throughput genomic editing in Escherichia coli.Appl Environ Microbiol. 2025 Jul 23;91(7):e0019325. doi: 10.1128/aem.00193-25. Epub 2025 Jun 12. Appl Environ Microbiol. 2025. PMID: 40503884 Free PMC article.
-
Bacterial genome engineering using CRISPR-associated transposases.Nat Protoc. 2024 Mar;19(3):752-790. doi: 10.1038/s41596-023-00927-3. Epub 2024 Jan 12. Nat Protoc. 2024. PMID: 38216671 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

