A Host Cell Vector Model for Analyzing Viral Protective Antigens and Host Immunity
- PMID: 40806621
- PMCID: PMC12347372
- DOI: 10.3390/ijms26157492
A Host Cell Vector Model for Analyzing Viral Protective Antigens and Host Immunity
Abstract
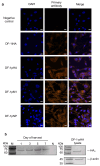
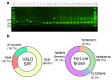
Avian influenza A viruses (IAVs) pose a persistent threat to the poultry industry, causing substantial economic losses. Although traditional vaccines have helped reduce the disease burden, they typically rely on multivalent antigens, emphasize humoral immunity, and require intensive production. This study aimed to establish a genetically matched host-cell system to evaluate antigen-specific immune responses and identify conserved CD8+ T cell epitopes in avian influenza viruses. To this end, we developed an MHC class I genotype (B21)-matched host (Lohmann VALO SPF chicken) and cell vector (DF-1 cell line) model. DF-1 cells were engineered to express the hemagglutinin (HA) gene of clade 2.3.4.4b H5N1 either transiently or stably, and to stably express the matrix 1 (M1) and nucleoprotein (NP) genes of A/chicken/South Korea/SL20/2020 (H9N2, Y280-lineage). Following prime-boost immunization with HA-expressing DF-1 cells, only live cells induced strong hemagglutination inhibition (HI) and virus-neutralizing (VN) antibody titers in haplotype-matched chickens. Importantly, immunization with DF-1 cells transiently expressing NP induced stronger IFN-γ production than those expressing M1, demonstrating the platform's potential for differentiating antigen-specific cellular responses. CD8+ T cell epitope mapping by mass spectrometry identified one distinct MHC class I-bound peptide from each of the HA-, M1-, and NP-expressing DF-1 cell lines. Notably, the identified HA epitope was conserved in 97.6% of H5-subtype IAVs, and the NP epitope in 98.5% of pan-subtype IAVs. These findings highlight the platform's utility for antigen dissection and rational vaccine design. While limited by MHC compatibility, this approach enables identification of naturally presented epitopes and provides insight into conserved, functionally constrained viral targets.
Keywords: CD8+ T cell epitope; cell vector; chicken; humoral immunity; influenza A virus; vaccine.
Conflict of interest statement
The author Hyuk-Joon Kwon was employed by GeNiner Inc. but has no potential interest relationship. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Comparative pathogenicity of three A(H5N1) clade 2.3.4.4b HPAI viruses in blue-winged teal and transmission to domestic poultry.mSphere. 2025 Jun 25;10(6):e0002125. doi: 10.1128/msphere.00021-25. Epub 2025 May 22. mSphere. 2025. PMID: 40401902 Free PMC article.
-
Greater Breadth of Vaccine-Induced Immunity in Females than Males Is Mediated by Increased Antibody Diversity in Germinal Center B Cells.mBio. 2022 Aug 30;13(4):e0183922. doi: 10.1128/mbio.01839-22. Epub 2022 Jul 20. mBio. 2022. PMID: 35856618 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Dynamics of a Panzootic: Genomic Insights, Host Range, and Epidemiology of the Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b in the United States.Viruses. 2025 Feb 25;17(3):312. doi: 10.3390/v17030312. Viruses. 2025. PMID: 40143242 Free PMC article. Review.
References
-
- Food and Agriculture Organization of the United Nations FAOSTAT. [(accessed on 22 February 2025)]. Available online: https://www.fao.org/faostat/en/#data/QCL.
-
- Pulit-Penaloza J.A., Brock N., Belser J.A., Sun X., Pappas C., Kieran T.J., Basu Thakur P., Zeng H., Cui D., Frederick J., et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg. Microbes Infect. 2024;13:2332667. doi: 10.1080/22221751.2024.2332667. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

