The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration
- PMID: 40806624
- PMCID: PMC12347410
- DOI: 10.3390/ijms26157498
The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration
Abstract
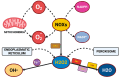
Oxidative stress is a defining and pervasive driver of neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). As a molecular accelerant, reactive oxygen species (ROS) and reactive nitrogen species (RNS) compromise mitochondrial function, amplify lipid peroxidation, induce protein misfolding, and promote chronic neuroinflammation, creating a positive feedback loop of neuronal damage and cognitive decline. Despite its centrality in promoting disease progression, attempts to neutralize oxidative stress with monotherapeutic antioxidants have largely failed owing to the multifactorial redox imbalance affecting each patient and their corresponding variation. We are now at the threshold of precision redox medicine, driven by advances in syndromic multi-omics integration, Artificial Intelligence biomarker identification, and the precision of patient-specific therapeutic interventions. This paper will aim to reveal a mechanistically deep assessment of oxidative stress and its contribution to diseases of neurodegeneration, with an emphasis on oxidatively modified proteins (e.g., carbonylated tau, nitrated α-synuclein), lipid peroxidation biomarkers (F2-isoprostanes, 4-HNE), and DNA damage (8-OHdG) as significant biomarkers of disease progression. We will critically examine the majority of clinical trial studies investigating mitochondria-targeted antioxidants (e.g., MitoQ, SS-31), Nrf2 activators (e.g., dimethyl fumarate, sulforaphane), and epigenetic reprogramming schemes aiming to re-establish antioxidant defenses and repair redox damage at the molecular level of biology. Emerging solutions that involve nanoparticles (e.g., antioxidant delivery systems) and CRISPR (e.g., correction of mutations in SOD1 and GPx1) have the potential to transform therapeutic approaches to treatment for these diseases by cutting the time required to realize meaningful impacts and meaningful treatment. This paper will argue that with the connection between molecular biology and progress in clinical hyperbole, dynamic multi-targeted interventions will define the treatment of neurodegenerative diseases in the transition from disease amelioration to disease modification or perhaps reversal. With these innovations at our doorstep, the future offers remarkable possibilities in translating network-based biomarker discovery, AI-powered patient stratification, and adaptive combination therapies into individualized/long-lasting neuroprotection. The question is no longer if we will neutralize oxidative stress; it is how likely we will achieve success in the new frontier of neurodegenerative disease therapies.
Keywords: Nrf2 pathway; antioxidant therapies; lipid peroxidation; mitochondrial dysfunction; multi-omics integration; neurodegenerative diseases; oxidative stress; precision redox medicine; protein carbonylation; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Precision Neuro-Oncology in Glioblastoma: AI-Guided CRISPR Editing and Real-Time Multi-Omics for Genomic Brain Surgery.Int J Mol Sci. 2025 Jul 30;26(15):7364. doi: 10.3390/ijms26157364. Int J Mol Sci. 2025. PMID: 40806492 Free PMC article. Review.
-
MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols.Alzheimers Dement. 2021 Apr;17(4):704-715. doi: 10.1002/alz.12215. Epub 2021 Jan 21. Alzheimers Dement. 2021. PMID: 33480172 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Tissue Factor and Its Cerebrospinal Fluid Protein Profiles in Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1405-1416. doi: 10.3233/JPD-240115. J Parkinsons Dis. 2024. PMID: 39240648 Free PMC article.
References
-
- Rauf A., Khalil A.A., Awadallah S., Khan S.A., Abu-Izneid T., Kamran M., Hemeg H.A., Mubarak M.S., Khalid A., Wilairatana P. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—A review. Food Sci. Nutr. 2023;12:675–693. doi: 10.1002/fsn3.3784. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

