Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis
- PMID: 40806625
- PMCID: PMC12347759
- DOI: 10.3390/ijms26157497
Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis
Abstract
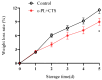
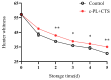
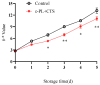
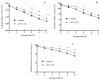
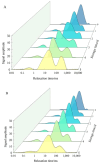
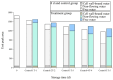
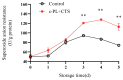
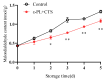
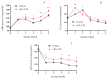
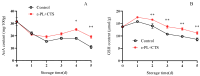
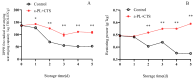
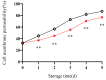
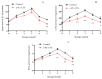
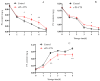
This study aimed to investigate the efficacy of a composite coating composed of 150 mg/L ε-Poly-L-lysine (ε-PL) and 5 g/L chitosan (CTS) in extending the shelf life and maintaining the postharvest quality of fresh Tremella fuciformis. Freshly harvested T. fuciformis were treated by surface spraying, with distilled water serving as the control. The effects of the coating on storage quality, physicochemical properties, reactive oxygen species (ROS) metabolism, and membrane lipid metabolism were evaluated during storage at (25 ± 1) °C. The results showed that the ε-PL/CTS composite coating significantly retarded quality deterioration, as evidenced by reduced weight loss, maintained whiteness and color, and higher retention of soluble sugars, soluble solids, and soluble proteins. The coating also effectively limited water migration and loss. Mechanistically, the coated T. fuciformis exhibited enhanced antioxidant capacity, characterized by increased superoxide anion (O2-) resistance capacity, higher activities of antioxidant enzymes (SOD, CAT, APX), and elevated levels of non-enzymatic antioxidants (AsA, GSH). This led to a significant reduction in malondialdehyde (MDA) accumulation, alongside improved DPPH radical scavenging activity and reducing power. Furthermore, the ε-PL/CTS coating preserved cell membrane integrity by inhibiting the activities of lipid-degrading enzymes (lipase, LOX, PLD), maintaining higher levels of key phospholipids (phosphatidylinositol and phosphatidylcholine), delaying phosphatidic acid accumulation, and consequently reducing cell membrane permeability. In conclusion, the ε-PL/CTS composite coating effectively extends the shelf life and maintains the quality of postharvest T. fuciformis by modulating ROS metabolism and preserving membrane lipid homeostasis. This study provides a theoretical basis and a practical approach for the quality control of fresh T. fuciformis.
Keywords: Tremella fuciformis; chitosan; membrane lipid metabolism; reactive oxygen species metabolism; ε-Poly-L-lysine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Investigating the Respiratory and Energy Metabolism Mechanisms behind ε-Poly-L-lysine Chitosan Coating's Improved Preservation Effectiveness on Tremella fuciformis.Foods. 2024 Feb 26;13(5):707. doi: 10.3390/foods13050707. Foods. 2024. PMID: 38472821 Free PMC article.
-
Application of Citral Nanoemulsion to Enhance Physicochemical Properties and Extend the Shelf Life of Fresh-Cut Longkong during Low-Temperature Storage.ACS Omega. 2025 Jun 23;10(26):27920-27935. doi: 10.1021/acsomega.5c01087. eCollection 2025 Jul 8. ACS Omega. 2025. PMID: 40657094 Free PMC article.
-
L-Phenylalanine preharvest spraying effectively enhances the reactive oxygen metabolism and antioxidant capacity in postharvest broccoli.Plant Physiol Biochem. 2025 Jun 23;227:110185. doi: 10.1016/j.plaphy.2025.110185. Online ahead of print. Plant Physiol Biochem. 2025. PMID: 40580791
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Antioxidant supplementation for sickle cell disease.Cochrane Database Syst Rev. 2024 May 22;5(5):CD013590. doi: 10.1002/14651858.CD013590.pub2. Cochrane Database Syst Rev. 2024. PMID: 38775255 Free PMC article.
References
-
- Qiang Y., He M., Zhang S., Su Y., Lin S., Guo Z., Zeng S., Zheng B. Pressure-controlled steam explosion as pretreatment for efficient extraction of T. fuciformis polysaccharide: Structure and bioactivity. Pt 2Int. J. Biol. Macromol. 2024;280:135766. doi: 10.1016/j.ijbiomac.2024.135766. - DOI - PubMed
-
- Lin Y., Lai D., Wang D., Zhou F., Tan B.K., Zhang Z., Hu J., Lin S. Application of curcumin-mediated antibacterial photodynamic technology for preservation of fresh T. fuciformis. LWT-Food Sci. Technol. 2021;147:111657. doi: 10.1016/j.lwt.2021.111657. - DOI
-
- Hu J., Liang M., Xian Y., Chen R., Wang L., Hou X., Wu Y. Development and validation of a multianalyte method for quantification of aflatoxins and bongkrekic acid in rice and noodle products using PRiME-UHPLC-MS/MS method. Food Chem. 2022;395:133598. doi: 10.1016/j.foodchem.2022.133598. - DOI - PubMed
Grants and funding
- ZYTS202417/Fujian Academy of Agricultural Sciences - Exploration Science and Technology Innovation Project
- 2024YFD2100803/National Key Research and Develop-ment Program of China
- Minnongkejiao (2024) - grant number 20/Fujian Province Modern Edible Fungus Industry Technology System Construction Project
- XTCXGC2021014/Fujian Pro-vincial People's Government-China Academy of Agricultural Sciences High-quality Development of Agriculture Beyond the "5511" Collaborative Innovation Engineering Project
- 2023J01377/Natural Science Foundation of Fujian Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

