Pathophysiology of Status Epilepticus Revisited
- PMID: 40806628
- PMCID: PMC12347885
- DOI: 10.3390/ijms26157502
Pathophysiology of Status Epilepticus Revisited
Abstract
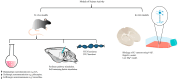
Status epilepticus occurs when a seizure lasts more than five minutes or when multiple seizures occur with incomplete return to baseline. SE induces a myriad of pathological changes involving synaptic and extra-synaptic factors. The transition from a self-limiting seizure to a self-sustaining one is established by maladaptive receptor trafficking, whereby GABAA receptors are progressively endocytosed while glutamatergic receptors (NMDA and AMPA) are transported to the synaptic membrane, causing excitotoxicity and alteration in glutamate-dependent downstream signaling. The subsequent influx of Ca2+ exposes neurons to increased levels of [Ca2+]i, which overwhelms mitochondrial buffering, resulting in irreversible mitochondrial membrane depolarization and mitochondrial injury. Oxidative stress resulting from mitochondrial leakage and increased production of reactive oxygen species activates the inflammasome and induces a damage-associated molecular pattern. Neuroinflammation perpetuates oxidative stress and exacerbates mitochondrial injury, thereby jeopardizing mitochondrial energy supply in a state of accelerated ATP consumption. Additionally, Ca2+ overload can directly damage neurons by activating enzymes involved in the breakdown of proteins, phospholipids, and nucleic acids. The cumulative effect of these effector pathways is neuronal injury and neuronal death. Surviving neurons undergo long-term alterations that serve as a substrate for epileptogenesis. This review highlights the multifaceted mechanisms underlying SE self-sustainability, pharmacoresistance, and subsequent epileptogenesis.
Keywords: epileptogenesis; pathophysiology; pharmacoresistance; status epilepticus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Alterations in GABAA receptor-mediated inhibition triggered by status epilepticus and their role in epileptogenesis and increased anxiety.Neurobiol Dis. 2024 Oct 1;200:106633. doi: 10.1016/j.nbd.2024.106633. Epub 2024 Aug 6. Neurobiol Dis. 2024. PMID: 39117119 Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Mitochondria from huntington´s disease striatal astrocytes are hypermetabolic and compromise neuronal branching.Cell Commun Signal. 2025 Jul 16;23(1):341. doi: 10.1186/s12964-025-02341-6. Cell Commun Signal. 2025. PMID: 40671084 Free PMC article.
-
Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: A systematic review with meta-analysis.Epilepsy Behav. 2015 Aug;49:325-36. doi: 10.1016/j.yebeh.2015.02.030. Epub 2015 Mar 25. Epilepsy Behav. 2015. PMID: 25817929
-
Elbow Fractures Overview.2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28723005 Free Books & Documents.
References
-
- Dingledine R., Varvel N.H., Dudek F.E. When and How Do Seizures Kill Neurons, and Is Cell Death Relevant to Epileptogenesis? In: Scharfman H.E., Buckmaster P.S., editors. Issues in Clinical Epileptology: A View from the Bench. Springer; Dordrecht, The Netherlands: 2014. pp. 109–122. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

