CRISPR-Cas Gene Editing Technology in Potato
- PMID: 40806631
- PMCID: PMC12347620
- DOI: 10.3390/ijms26157496
CRISPR-Cas Gene Editing Technology in Potato
Abstract
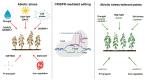
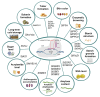
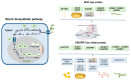
Potato (Solanum tuberosum L.) is one of the most important food crops in the world, ranking fourth after rice, maize, and wheat. Potatoes are exposed to biotic and abiotic environmental factors, which lead to economic losses and increase the possibility of food security threats in many countries. Traditional potato breeding faces several challenges, primarily due to its genetic complexity and the time-consuming nature of the process. Therefore, gene editing-CRISPR-Cas technology-allows for more precise and rapid changes to the potato genome, which can speed up the breeding process and lead to more effective varieties. In this review, we consider CRISPR-Cas technology as a potential tool for plant breeding strategies to ensure global food security. This review summarizes in detail current and potential technological breakthroughs that open new opportunities for the use of CRISPR-Cas technology for potato breeding, as well as for increasing resistance to abiotic and biotic stresses, and improving potato tuber quality. In addition, the review discusses the challenges and future perspectives of the CRISPR-Cas system in the prospects of the development of potato production and the regulation of gene-edited crops in different countries around the world.
Keywords: CRISPR-Cas; abiotic stress; biotic stress; disease resistance; drought; gene editing; improve breeding process; potato; tuber quality; viral resistance.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this review, in the collection of data, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish this review.
Figures


Similar articles
-
Harnessing CRISPR/Cas9 in engineering biotic stress immunity in crops.Planta. 2025 Jul 15;262(3):54. doi: 10.1007/s00425-025-04769-z. Planta. 2025. PMID: 40663257 Review.
-
Enhancing the quality of staple food crops through CRISPR/Cas-mediated site-directed mutagenesis.Planta. 2023 Mar 13;257(4):78. doi: 10.1007/s00425-023-04110-6. Planta. 2023. PMID: 36913066 Review.
-
CRISPR/Cas genome editing in soybean: challenges and new insights to overcome existing bottlenecks.J Adv Res. 2025 Jul;73:53-72. doi: 10.1016/j.jare.2024.08.024. Epub 2024 Aug 18. J Adv Res. 2025. PMID: 39163906 Free PMC article. Review.
-
CRISPR/Cas system-mediated transgene-free or DNA-free genome editing in plants.Theor Appl Genet. 2025 Aug 12;138(9):210. doi: 10.1007/s00122-025-04990-0. Theor Appl Genet. 2025. PMID: 40794116 Review.
-
Biotechnological breakthroughs in rice disease management: an overview from transgenics to CRISPR.Mol Biol Rep. 2025 Jun 20;52(1):616. doi: 10.1007/s11033-025-10701-1. Mol Biol Rep. 2025. PMID: 40540136 Review.
References
-
- Sapakhova Z., Abilda Z., Toishimanov M., Daurov D., Daurova A., Raissova N., Sidorik A., Kanat R., Zhambakin K., Shamekova M. Early Generation Selection of Potato Breeding Lines. Horticulturae. 2024;10:1121. doi: 10.3390/horticulturae10101121. - DOI
-
- Daurov D., Daurova A., Sapakhova Z., Kanat R., Akhmetzhanova D., Abilda Z., Toishimanov M., Raissova N., Otynshiyev M., Zhambakin K., et al. The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy. 2024;14:1782. doi: 10.3390/agronomy14081782. - DOI
-
- Campos H., Ortiz O. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind. Springer International Publishing; Cham, Switzerland: 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

