Make Acetylcholine Great Again! Australian Skinks Evolved Multiple Neurotoxin-Proof Nicotinic Acetylcholine Receptors in Defiance of Snake Venom
- PMID: 40806638
- PMCID: PMC12347452
- DOI: 10.3390/ijms26157510
Make Acetylcholine Great Again! Australian Skinks Evolved Multiple Neurotoxin-Proof Nicotinic Acetylcholine Receptors in Defiance of Snake Venom
Abstract
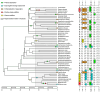
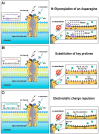
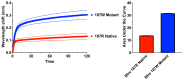
Many vertebrates have evolved resistance to snake venom as a result of coevolutionary chemical arms races. In Australian skinks (family Scincidae), who often encounter venomous elapid snakes, the frequency, diversity, and molecular basis of venom resistance have been unexplored. This study investigated the evolution of neurotoxin resistance in Australian skinks, focusing on mutations in the muscle nicotinic acetylcholine receptor (nAChR) α1 subunit's orthosteric site that prevent pathophysiological binding by α-neurotoxins. We sampled a broad taxonomic range of Australian skinks and sequenced the nAChR α1 subunit gene. Key resistance-conferring mutations at the toxin-binding site (N-glycosylation motifs, proline substitutions, arginine insertions, changes in the electrochemical state of the receptor, and novel cysteines) were identified and mapped onto the skink organismal phylogeny. Comparisons with other venom-resistant taxa (amphibians, mammals, and reptiles) were performed, and structural modelling and binding assays were used to evaluate the impact of these mutations. Multiple independent origins of α-neurotoxin resistance were found across diverse skink lineages. Thirteen lineages evolved at least one resistance motif and twelve additional motifs evolved within these lineages, for a total of twenty-five times of α-neurotoxic venoms resistance. These changes sterically or electrostatically inhibit neurotoxin binding. Convergent mutations at the orthosteric site include the introduction of N-linked glycosylation sites previously known from animals as diverse as cobras and mongooses. However, an arginine (R) substitution at position 187 was also shown to have evolved on multiple occasions in Australian skinks, a modification previously shown to be responsible for the Honey Badger's iconic resistance to cobra venom. Functional testing confirmed this mode of resistance in skinks. Our findings reveal that venom resistance has evolved extensively and convergently in Australian skinks through repeated molecular adaptations of the nAChR in response to the enormous selection pressure exerted by elapid snakes subsequent to their arrival and continent-wide dispersal in Australia. These toxicological findings highlight a remarkable example of convergent evolution across vertebrates and provide insight into the adaptive significance of toxin resistance in snake-lizard ecological interactions.
Keywords: adaptation; evolution; neurotoxin; nicotinic acetylcholine receptor; skink; venom.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Widespread Evolution of Molecular Resistance to Snake Venom α-Neurotoxins in Vertebrates.Toxins (Basel). 2020 Oct 2;12(10):638. doi: 10.3390/toxins12100638. Toxins (Basel). 2020. PMID: 33023159 Free PMC article.
-
Survivor, family and professional experiences of psychosocial interventions for sexual abuse and violence: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2022 Oct 4;10(10):CD013648. doi: 10.1002/14651858.CD013648.pub2. Cochrane Database Syst Rev. 2022. PMID: 36194890 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Fry B.G., Roelants K., Champagne D.E., Scheib H., Tyndall J.D., King G.F., Nevalainen T.J., Norman J.A., Lewis R.J., Norton R.S., et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

