Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes
- PMID: 40806667
- PMCID: PMC12347949
- DOI: 10.3390/ijms26157539
Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes
Abstract
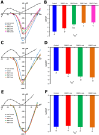
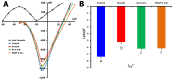
L-type Ca2+ channels, particularly CaV1.2, play a crucial role in cardiac excitation-contraction coupling and are known to exhibit mechanosensitivity. However, the mechanisms regulating their response to mechanical stress remain poorly understood. To investigate the mechanosensitivity and nitric oxide (NO)-dependent regulation of L-type Ca2+ channels in rat ventricular cardiomyocytes, we used RNA sequencing to assess isoform expression and whole-cell patch-clamp recordings to measure L-type Ca2+ current (ICa,L) under controlled mechanical and pharmacological conditions. RNA sequencing revealed predominant expression of CaV1.2 (TPM: 0.1170 ± 0.0075) compared to CaV1.3 (0.0021 ± 0.0002) and CaV1.1 (0.0002 ± 0.0002). Local axial stretch (6-10 μm) consistently reduced ICa,L in proportion to stretch magnitude. The NO donor SNAP (200 μM) had variable effects on basal ICa,L in unstretched cells (stimulatory, inhibitory, or biphasic) but consistently restored stretch-reduced ICa,L to control levels. Ascorbic acid (10 μM), which reduces S-nitrosylation, increased basal ICa,L and partially restored the reduction caused by stretch, implicating S-nitrosylation in channel regulation. The sGC inhibitor ODQ (5 μM) decreased ICa,L in both stretched and unstretched cells, indicating involvement of the NO-cGMP pathway. Mechanical stress modulates L-type Ca2+ channels through a complex interplay between S-nitrosylation and NO-cGMP signaling, with S-nitrosylation playing a predominant role in stretch-induced effects. This mechanism may represent a key component of cardiac mechanotransduction and could be relevant for therapeutic targeting in cardiac pathologies involving mechanically induced dysfunction.
Keywords: L-type Ca2+ channels; NO-cGMP; S-nitrosylation; cardiomyocytes; mechanosensitivity; nitric oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes.Int J Mol Sci. 2025 Jul 11;26(14):6653. doi: 10.3390/ijms26146653. Int J Mol Sci. 2025. PMID: 40724903 Free PMC article.
-
A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current.Cells. 2025 Aug 8;14(16):1226. doi: 10.3390/cells14161226. Cells. 2025. PMID: 40862705 Free PMC article.
-
The role of activation of two different sGC binding sites by NO-dependent and NO-independent mechanisms in the regulation of SACs in rat ventricular cardiomyocytes.Physiol Rep. 2022 Apr;10(7):e15246. doi: 10.14814/phy2.15246. Physiol Rep. 2022. PMID: 35384354 Free PMC article.
-
S-nitrosylation of cardiac myocyte proteins may underlie sex differences in cardiac disease.Front Physiol. 2025 May 6;16:1565917. doi: 10.3389/fphys.2025.1565917. eCollection 2025. Front Physiol. 2025. PMID: 40395646 Free PMC article. Review.
-
Inhaled nitric oxide for treating pain crises in people with sickle cell disease.Cochrane Database Syst Rev. 2022 Jul 8;7(7):CD011808. doi: 10.1002/14651858.CD011808.pub3. Cochrane Database Syst Rev. 2022. PMID: 35802341 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

