Promoter H3K4me3 and Gene Expression Involved in Systemic Metabolism Are Altered in Fetal Calf Liver of Nutrient-Restricted Dams
- PMID: 40806668
- PMCID: PMC12347666
- DOI: 10.3390/ijms26157540
Promoter H3K4me3 and Gene Expression Involved in Systemic Metabolism Are Altered in Fetal Calf Liver of Nutrient-Restricted Dams
Abstract
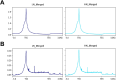
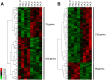
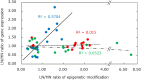
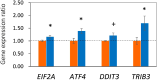
Maternal undernutrition (MUN) causes severe metabolic disruption in the offspring of mammals. Here we determined the role of histone modification in hepatic gene expression in late-gestation fetuses of nutritionally restricted cows, an established model using low-nutrition (LN) and high-nutrition (HN) conditions. The chromatin immunoprecipitation sequencing results show that genes with an altered trimethylation of histone 3 lysine 4 (H3K4me3) are associated with cortisol synthesis and secretion, the PPAR signaling pathway, and aldosterone synthesis and secretion. Genes with the H3K27me3 alteration were associated with glutamatergic synapse and gastric acid secretion. Compared to HN fetuses, promoter H3K4me3 levels in LN fetuses were higher in GDF15, IRF2BP2, PPP1R3B, and QRFPR but lower in ANGPTL4 and APOA5. Intriguingly, genes with the greatest expression changes (>1.5-fold) exhibited the anticipated up-/downregulation from elevated or reduced H3K4me3 levels; however, a significant relationship was not observed between promoter CpG methylation or H3K27me3 and the gene set with the greatest expression changes. Furthermore, the stress response genes EIF2A, ATF4, DDIT3, and TRIB3 were upregulated in the MUN fetal liver, suggesting involvement of the response in GDF15 activation. Thus, H3K4me3 likely plays a crucial role in MUN-induced physiological adaptation, altering the hepatic gene expression responsible for the integrated stress response and systemic energy metabolism, especially circulating lipoprotein lipase regulation.
Keywords: epigenetics; fetal growth restriction; histone modification; integrated stress response; liver; maternal undernutrition; metabolic programming.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Govoni K.E., Reed S.A., Zinn S.A. Cell Biology Symposium: Metabolic Responses to Stress: From Animal to Cell: Poor maternal nutrition during gestation: Effects on offspring whole-body and tissue-specific metabolism in livestock species1,2. J. Anim. Sci. 2019;97:3142–3152. doi: 10.1093/jas/skz157. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

