DHA-Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling
- PMID: 40806677
- PMCID: PMC12347805
- DOI: 10.3390/ijms26157549
DHA-Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling
Abstract
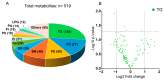
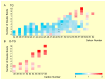
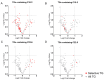
Tacrolimus (TAC)-induced chronic nephrotoxicity (TAC nephrotoxicity) remains a major contributor to late allograft dysfunction in kidney transplant recipients. Although detailed mechanisms remain incompletely understood, our previous metabolomic studies revealed disruptions in carnitine-related and redox pathways, suggesting impaired mitochondrial β-oxidation of fatty acids. To further characterize metabolic alterations associated with this condition, we conducted an untargeted lipidomic analysis of renal tissues using a murine model of TAC nephrotoxicity. TAC (1 mg/kg/day) or saline was subcutaneously administered to male ICR mice for 28 days, and kidney tissues were harvested for comprehensive lipidomic profiling. Lipidomic analysis was performed with liquid chromatography-tandem mass spectrometry (p < 0.05, n = 5/group). Triacylglycerols (TGs) were the predominant lipid class identified. TAC-treated mice exhibited reduced levels of unsaturated TG species with low carbon numbers, whereas TGs with higher carbon numbers and various degrees of unsaturation were increased. All detected TGs containing docosahexaenoic acid (DHA) showed an increasing trend in TAC-treated kidneys. Although accumulation of polyunsaturated TGs has been previously observed in chronic kidney disease, the preferential increase in DHA-containing TGs appears to be a unique feature of TAC-induced nephrotoxicity. These results suggest that DHA-enriched TGs may serve as a metabolic signature of TAC nephrotoxicity and offer new insights into its pathophysiology.
Keywords: calcineurin inhibitor; docosahexaenoic acid; lipidomics; renal injury; tacrolimus; triacylglycerol profiling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Baumgart D.C., Pintoffl J.P., Sturm A., Wiedenmann B., Dignass A.U. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up. Am. J. Gastroenterol. 2006;101:1048–1056. doi: 10.1111/j.1572-0241.2006.00524.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

