Bidirectional Interplay Between Microglia and Mast Cells
- PMID: 40806683
- PMCID: PMC12347062
- DOI: 10.3390/ijms26157556
Bidirectional Interplay Between Microglia and Mast Cells
Abstract
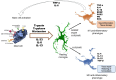
Microglia, the brain's resident innate immune cells, play a fundamental role in maintaining neural homeostasis and mediating responses to injury or infection. Upon activation, microglia undergo morphological and functional changes, including phenotypic switching between pro- and anti-inflammatory types and the release of different inflammatory mediators. These processes contribute to neuroprotection and the pathogenesis of various central nervous system (CNS) disorders. Mast cells, although sparsely located in the brain, exert a significant influence on neuroinflammation through their interactions with microglia. Through degranulation and secretion of different mediators, mast cells disrupt the blood-brain barrier and modulate microglial responses, including alteration of microglial phenotypes. Notably, mast cell-derived factors, such as histamine, interleukins, and tryptase, activate microglia through various pathways including protease-activated receptor 2 and purinergic receptors. These interactions amplify inflammatory cascades via various signaling pathways. Previous studies have revealed an exceedingly complex crosstalk between mast cells and microglia suggesting a bidirectional regulation of CNS immunity, implicating their cooperation in both neurodegenerative progression and repair mechanisms. Here, we review some of the diverse communication pathways involved in this complex interplay. Understanding this crosstalk may offer novel insights into the cellular dynamics of neuroinflammation and highlight potential therapeutic targets for a variety of CNS disorders.
Keywords: PAR2; cytokines; histamine; mast cells; microglia; neuroinflammation; purinergic receptors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Histamine as a mediator of cross-talk between human lung mast cells and macrophages.Sci Rep. 2025 Aug 30;15(1):31969. doi: 10.1038/s41598-025-17262-0. Sci Rep. 2025. PMID: 40885833 Free PMC article.
-
Microglial activation states and their implications for Alzheimer's Disease.J Prev Alzheimers Dis. 2025 Jan;12(1):100013. doi: 10.1016/j.tjpad.2024.100013. Epub 2025 Jan 1. J Prev Alzheimers Dis. 2025. PMID: 39800461 Free PMC article. Review.
-
The role of mast cells in allergic rhinitis.PeerJ. 2025 Jul 30;13:e19734. doi: 10.7717/peerj.19734. eCollection 2025. PeerJ. 2025. PMID: 40755802 Free PMC article. Review.
References
-
- Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., Császár E., Fekete R., West B.L., Katona G., et al. Microglia Protect against Brain Injury and Their Selective Elimination Dysregulates Neuronal Network Activity after Stroke. Nat. Commun. 2016;7:11499. doi: 10.1038/ncomms11499. - DOI - PMC - PubMed
-
- Ghimire A., Rehman S.A., Subhani A., Khan M.A., Rahman Z., Iqubal M.K., Iqubal A. Mechanism of Microglia-Mediated Neuroinflammation, Associated Cognitive Dysfunction, and Therapeutic Updates in Alzheimer’s Disease. hLife. 2025;3:64–81. doi: 10.1016/j.hlife.2024.11.006. - DOI
-
- Tsai M., Grimbaldeston M., Galli S. Madame Curie Bioscience Database. Landes Bioscience; Austin, TX, USA: 2000. Mast Cells and Immunoregulation/Immunomodulation.
-
- Conti P., Lauritano D., Caraffa A., Gallenga C.E., Kritas S.K., Ronconi G., Martinotti S. Microglia and Mast Cells Generate Proinflammatory Cytokines in the Brain and Worsen Inflammatory State: Suppressor Effect of IL-37. Eur. J. Pharmacol. 2020;875:173035. doi: 10.1016/j.ejphar.2020.173035. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

