Lycopene Inhibits PRRSV Replication by Suppressing ROS Production
- PMID: 40806687
- PMCID: PMC12347652
- DOI: 10.3390/ijms26157560
Lycopene Inhibits PRRSV Replication by Suppressing ROS Production
Abstract
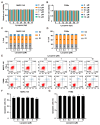
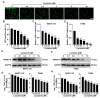
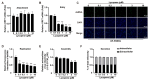
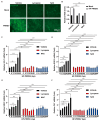
Porcine reproductive and respiratory syndrome virus (PRRSV), an enveloped single-stranded positive-sense RNA virus, poses a significant threat to global swine production. Despite the availability of modified live virus and inactivated vaccines, their limited efficacy and safety concerns highlight the urgent need for novel antiviral therapeutics. This study aimed to investigate the molecular mechanisms by which lycopene inhibits PRRSV replication. Initial assessments confirmed that lycopene did not adversely affect cellular viability, cell cycle progression, or apoptosis. Using fluorescence microscopy, flow cytometry, immunoblotting, quantitative real-time PCR (qRT-PCR), and viral titration assays, lycopene was shown to exhibit potent antiviral activity against PRRSV. Mechanistic studies revealed that lycopene suppresses reactive oxygen species (ROS) production, which is critical for PRRSV proliferation. Additionally, lycopene attenuated PRRSV-induced inflammatory responses, as demonstrated by immunoblotting, ELISA, and qRT-PCR assays. These findings suggest that lycopene inhibits PRRSV replication by modulating ROS levels and mitigating inflammation, offering a promising avenue for the development of antiviral therapeutics. This study provides new insights and strategies for combating PRRSV infections, emphasizing the potential of lycopene as a safe and effective antiviral agent.
Keywords: PRRSV; ROS; inflammation; lycopene.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Self-assembled cyanidin-3-O-glucoside nanoparticles alleviate inflammation and ferroptosis induced by PRRSV infection.J Virol. 2025 Aug 14:e0095425. doi: 10.1128/jvi.00954-25. Online ahead of print. J Virol. 2025. PMID: 40810536
-
The mRNA and protein of IL-8 oppositely regulate PRRSV replication via nucleoprotein and 14-3-3γ.J Virol. 2025 Aug 19;99(8):e0065525. doi: 10.1128/jvi.00655-25. Epub 2025 Jul 24. J Virol. 2025. PMID: 40704900 Free PMC article.
-
An attenuated herpesvirus vectored vaccine candidate induces T-cell responses against highly conserved porcine reproductive and respiratory syndrome virus M and NSP5 proteins that are unable to control infection.Front Immunol. 2023 Aug 3;14:1201973. doi: 10.3389/fimmu.2023.1201973. eCollection 2023. Front Immunol. 2023. PMID: 37600784 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., McCullough S., Morrison R.B., Joo H.S., et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. - DOI - PubMed
-
- Baron T., Albina E., Leforban Y., Madec F., Guilmoto H., Plana Duran J., Vannier P. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann. Rech. Vet. 1992;23:161–166. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

