A Bifunctional Anti-PD-1/TGF-β Fusion Antibody Restores Antitumour Immunity and Remodels the Tumour Microenvironment
- PMID: 40806691
- PMCID: PMC12347602
- DOI: 10.3390/ijms26157567
A Bifunctional Anti-PD-1/TGF-β Fusion Antibody Restores Antitumour Immunity and Remodels the Tumour Microenvironment
Abstract
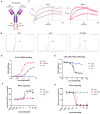
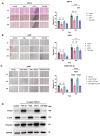
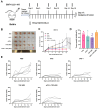
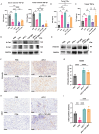
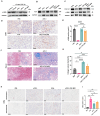
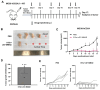
Although PD-1/PD-L1 inhibitors have transformed cancer immunotherapy, a substantial proportion of patients derive no clinical benefit due to resistance driven by the tumour microenvironment (TME). Transforming growth factor-β (TGF-β) is a key immunosuppressive cytokine implicated in this resistance. Several bifunctional antibodies that co-target PD-1 and TGF-β signalling have entered clinical trials and shown encouraging efficacy, but the mechanistic basis of their synergy is not fully understood. Here, we engineered 015s, a bifunctional fusion antibody that simultaneously targets murine PD-1 and TGF-β and evaluated its antitumour efficacy and mechanistic impact in pre-clinical models. Antibody 015s exhibited high affinity, dual target binding, and the effective inhibition of PD-1 and TGF-β signalling. In vivo, 015s significantly suppressed tumour growth compared with anti-mPD-1 or TGF-β receptor II (TGF-βRII) monotherapy. When combined with the CD24-targeted ADC, 015s produced even greater antitumour activity and achieved complete tumour regression. Mechanistic studies demonstrated that 015s significantly reduced tumour cell migration and invasion, reversed epithelial-mesenchymal transition (EMT), decreased microvascular density, and attenuated collagen deposition within the TME. Antibody 015s also decreased bioactive TGF-β1 and increased intratumoural IFN-γ, creating a more immunostimulatory milieu. These findings support further development of PD-1/TGF-β bifunctional antibodies for cancers with high TGF-β activity or limited response to immune checkpoint blockade.
Keywords: PD-1; TGF-β; bifunctional fusion antibody; cancer immunotherapy.
Conflict of interest statement
Dr. Xiao Huang, Dr. Xiaoqiang Kang, and Dr. Hong Ling were employed by Nanjing Leads Biolabs Co., Ltd., Nanjing, China. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. They had no role in the study design; data collection, analysis, or interpretation; manuscript writing; or the decision to publish the results.
Figures
Similar articles
-
Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment.Mol Oncol. 2022 Jun;16(11):2117-2134. doi: 10.1002/1878-0261.13146. Epub 2022 Jan 4. Mol Oncol. 2022. PMID: 34854206 Free PMC article. Review.
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The pancreatic cancer immune tumor microenvironment is negatively remodeled by gemcitabine while TGF-β receptor plus dual checkpoint inhibition maintains antitumor immune cells.Mol Carcinog. 2022 Jun;61(6):549-557. doi: 10.1002/mc.23401. Epub 2022 Mar 23. Mol Carcinog. 2022. PMID: 35319799 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
Cited by
-
Tumor microenvironment-preserving gliosarcoma organoids as an in vitro preclinical platform: a comparative analysis with glioblastoma models.J Transl Med. 2025 Aug 14;23(1):915. doi: 10.1186/s12967-025-06952-y. J Transl Med. 2025. PMID: 40813987 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

