MCC950 Alleviates Fat Embolism-Induced Acute Respiratory Distress Syndrome Through Dual Modulation of NLRP3 Inflammasome and ERK Pathways
- PMID: 40806699
- PMCID: PMC12347069
- DOI: 10.3390/ijms26157571
MCC950 Alleviates Fat Embolism-Induced Acute Respiratory Distress Syndrome Through Dual Modulation of NLRP3 Inflammasome and ERK Pathways
Abstract
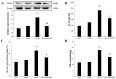
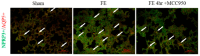
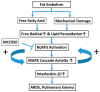
Fat embolism is a critical medical emergency often resulting from long bone fractures or amputations, leading to acute respiratory distress syndrome (ARDS). The NOD-like receptor pyrin domain-containing 3 (NLRP3) inflammasome, a key regulator of innate immunity, is activated by reactive oxygen species and tissue damage, contributing to inflammatory responses. This study examines the role of NLRP3 in fat embolism-induced ARDS and evaluates the therapeutic potential of MCC950, a selective NLRP3 antagonist. Fat embolism was induced by fatty micelle injection into the tail vein of Sprague Dawley rats. Pulmonary injury was assessed through lung weight gain as an edema indicator, NLRP3 expression via Western blot, and IL-1β levels using ELISA. Histological damage and macrophage infiltration were evaluated with hematoxylin and eosin staining. Fat embolism significantly increased pulmonary NLRP3 expression, lipid peroxidation, IL-1β release, and macrophage infiltration within four hours, accompanied by severe pulmonary edema. NLRP3 was localized in type I alveolar cells, co-localizing with aquaporin 5. Administration of MCC950 significantly reduced inflammatory responses, lipid peroxidation, pulmonary edema, and histological damage, while attenuating MAPK cascade phosphorylation of ERK and Raf. These findings suggest that NLRP3 plays a critical role in fat embolism-induced acute respiratory distress syndrome, and its inhibition by MCC950 may offer a promising therapeutic approach.
Keywords: MCC950; NOD-like receptor pyrin domain-containing 3 (NLRP3); acute respiratory distress syndrome (ARDS); fat embolism; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

