Structural Insights and Calcium-Switching Mechanism of Fasciola hepatica Calcium-Binding Protein FhCaBP4
- PMID: 40806711
- PMCID: PMC12347358
- DOI: 10.3390/ijms26157584
Structural Insights and Calcium-Switching Mechanism of Fasciola hepatica Calcium-Binding Protein FhCaBP4
Abstract
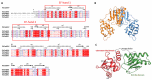
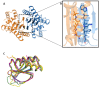
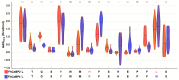
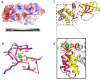
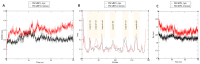
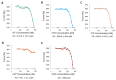
Fasciola hepatica remains a global health and economic concern, and treatment still relies heavily on triclabendazole. At the parasite-host interface, F. hepatica calcium-binding proteins (FhCaBPs) have a unique EF-hand/DLC-like domain fusion found only in trematodes. This makes it a parasite-specific target for small compounds and vaccinations. To enable novel therapeutic strategies, we report the first elevated-resolution structure of a full-length FhCaBP4. The apo structure was determined at 1.93 Å resolution, revealing a homodimer architecture that integrates an N-terminal, calmodulin-like, EF-hand pair with a C-terminal dynein light chain (DLC)-like domain. Structure-guided in silico mutagenesis identified a flexible, 16-residue β4-β5 loop (LTGSYWMKFSHEPFMS) with an FSHEPF core that demonstrates greater energetic variability than its FhCaBP2 counterpart, likely explaining the distinct ligand-binding profiles of these paralogs. Molecular dynamics simulations and AlphaFold3 modeling suggest that EF-hand 2 acts as the primary calcium-binding site, with calcium coordination inducing partial rigidification and modest expansion of the protein structure. Microscale thermophoresis confirmed calcium as the major ligand, while calmodulin antagonists bound with lower affinity and praziquantel demonstrated no interaction. Thermal shift assays revealed calcium-dependent stabilization and a merger of biphasic unfolding transitions. These results suggest that FhCaBP4 functions as a calcium-responsive signaling hub, with an allosterically coupled EF-hand-DLC interface that could serve as a structurally tractable platform for drug targeting in trematodes.
Keywords: DLC-like domain; EF-hand; Fasciola hepatica; FhCaBP4; calcium; homodimer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
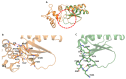
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
FhCaBP4: a Fasciola hepatica calcium-binding protein with EF-hand and dynein light chain domains.Parasitol Res. 2012 Oct;111(4):1707-13. doi: 10.1007/s00436-012-3010-y. Epub 2012 Jul 8. Parasitol Res. 2012. PMID: 22773043
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Beesley N.J., Cwiklinski K., Allen K., Hoyle R.C., Spithill T.W., La Course E.J., Williams D.J., Paterson S., Hodgkinson J.E. A major locus confers triclabendazole resistance in Fasciola hepatica and shows dominant inheritance. PLoS Pathog. 2023;19:e1011081. doi: 10.1371/journal.ppat.1011081. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

