Extracellular Adenosine in Gastric Cancer: The Role of GCSCs
- PMID: 40806724
- PMCID: PMC12347490
- DOI: 10.3390/ijms26157594
Extracellular Adenosine in Gastric Cancer: The Role of GCSCs
Abstract
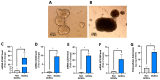
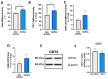
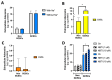
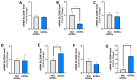
Gastric cancer (GC) is among the most common and deadliest types of cancer, with a poor prognosis primarily due to late-stage detection and the presence of cancer stem cells (CSCs). This study investigates the mechanisms regulating extracellular adenosine levels in gastric cancer stem-like cells (GCSCs) derived from the MKN-74 cell line. Our results show that GCSCs release more ATP into the extracellular medium and exhibit higher levels of CD39 expression, which enables them to hydrolyze a greater amount of ATP. Furthermore, we also found that GCSCs possess a greater capacity to hydrolyze AMP, primarily due to the activity of the CD73 protein, with no significant changes in CD73 transcripts and protein levels between GCSCs and differentiated cells. Additionally, adenosine transport is primarily mediated by members of the equilibrative nucleoside transporter (ENT) family in GCSCs, where a significant increase in the expression level of the ENT2 protein is observed compared to non-GCSCs MKN-74 cells. These findings suggest that targeting the adenosine metabolism pathway in GCSCs could be a potential therapeutic strategy for gastric cancer.
Keywords: adenosine; gastric cancer; gastric cancer stem-like cells (GCSCs); stomach cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
P2X7 a new therapeutic target to block vesicle-dependent metastasis in colon carcinoma: Role of the A2A/CD39/CD73 axis.Cell Death Dis. 2025 Aug 4;16(1):587. doi: 10.1038/s41419-025-07897-2. Cell Death Dis. 2025. PMID: 40759630 Free PMC article.
-
Purinergic signaling modulates CD4+ T cells with cytotoxic potential during Trypanosoma cruzi infection.J Clin Invest. 2025 Jul 1;135(13):e186785. doi: 10.1172/JCI186785. eCollection 2025 Jul 1. J Clin Invest. 2025. PMID: 40590226 Free PMC article.
-
TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells.Int J Mol Sci. 2025 Mar 7;26(6):2413. doi: 10.3390/ijms26062413. Int J Mol Sci. 2025. PMID: 40141056 Free PMC article.
-
Adenosine signaling in tumor-associated macrophages and targeting adenosine signaling for cancer therapy.Cancer Biol Med. 2024 Dec 3;21(11):995-1011. doi: 10.20892/j.issn.2095-3941.2024.0228. Cancer Biol Med. 2024. PMID: 39629670 Free PMC article. Review.
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
References
-
- Seeneevassen L., Zaafour A., Sifré E., Genevois C., Nguyen T.L., Pobiedonoscew Y., Giese A., Guignard J., Tiffon C., Rousseau B., et al. Targeting metastasis-initiating cancer stem cells in gastric cancer with leukaemia inhibitory factor. Cell Death Discov. 2024;10:120. doi: 10.1038/s41420-024-01839-1. - DOI - PMC - PubMed
-
- Mokadem I., Dijksterhuis W.P.M., van Putten M., Heuthorst L., de Vos-Geelen J.M., Haj Mohammad N., Nieuwenhuijzen G.A.P., van Laarhoven H.W.M., Verhoeven R.H.A. Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: A multicenter study. Gastric Cancer. 2019;22:1263–1273. doi: 10.1007/s10120-019-00956-6. - DOI - PMC - PubMed
-
- Iwu C.D., Iwu-Jaja C.J. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene. 2023;3:256–268. doi: 10.3390/hygiene3030019. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

