The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase
- PMID: 40806728
- PMCID: PMC12347091
- DOI: 10.3390/ijms26157599
The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase
Abstract
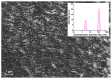
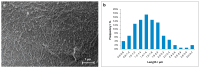
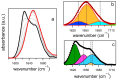
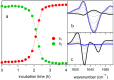
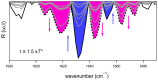
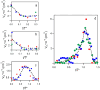
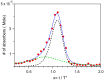
The kinetics of insulin aggregation and fibril formation were studied in vitro using Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FTIR) spectroscopy. Our investigation centered on the protein's morphological and structural changes to better understand the transient molecular configurations that occur during the lag phase. SEM images showed that, already at early incubation stages, a network of disordered pseudo-filaments, ranging in length between 200 and 500 nanometers, develops on the surface of large aggregates. At later stages, fibrils catalyzed by protein aggregates were observed. Principal Component Analysis (PCA) of the FTIR data identified signatures of intramolecular β-sheet secondary structures forming during the lag phase and at the onset of the exponential growth phase. These absorption bands are linked to secondary nucleation mechanisms due to their transient nature. This interpretation is further supported by a chemical equilibrium model, which yielded a reliable secondary nucleation rate constant, K2, on the order of 104 M-2 s-1.
Keywords: amyloid fibrils; insulin; lag phase; secondary nucleation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

