Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis
- PMID: 40806735
- PMCID: PMC12347807
- DOI: 10.3390/ijms26157611
Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis
Abstract
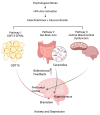
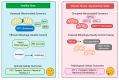
The relationship between metabolic dysfunction and mental health disorders is complex and has received increasing attention. This review integrates current research to explore how stress-related growth differentiation factor 15 (GDF15) signaling, ceramides derived from gut microbiota, and mitochondrial dysfunction in the brain interact to influence both metabolic and psychiatric conditions. Evidence suggests that these pathways converge to regulate brain energy homeostasis through feedback mechanisms involving the autonomic nervous system and the hypothalamic-pituitary-adrenal axis. GDF15 emerges as a key stress-responsive biomarker that links peripheral metabolism with brainstem GDNF family receptor alpha-like (GFRAL)-mediated anxiety circuits. Meanwhile, ceramides impair hippocampal mitochondrial function via membrane incorporation and disruption of the respiratory chain. These disruptions may contribute to sustained pathological states such as depression, anxiety, and cognitive dysfunction. Although direct mechanistic data are limited, integrating these pathways provides a conceptual framework for understanding metabolic-psychiatric comorbidities. Furthermore, differences in age, sex, and genetics may influence these systems, highlighting the need for personalized interventions. Targeting mitochondrial function, GDF15-GFRAL signaling, and gut microbiota composition may offer new therapeutic strategies. This integrative perspective helps conceptualize how metabolic and psychiatric mechanisms interact for understanding the pathophysiology of metabolic and psychiatric comorbidities and highlights therapeutic targets for precision medicine.
Keywords: GDF15; anxiety; biomarkers; depression; gut–brain axis; mental health; metabolism; mitochondria; precision medicine; stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Arnold M., Buyukozkan M., Doraiswamy P.M., Nho K., Wu T., Gudnason V., Launer L.J., Wang-Sattler R., Adamski J., Alzheimer’s Disease Neuroimaging I., et al. Individual bioenergetic capacity as a potential source of resilience to Alzheimer’s disease. medRxiv. 2024 doi: 10.1101/2024.01.23.23297820. - DOI - PMC - PubMed
-
- Mokhtari A., Ibrahim E.C., Gloaguen A., Barrot C.C., Cohen D., Derouin M., Vachon H., Charbonnier G., Loriod B., Decraene C., et al. Using multiomic integration to improve blood biomarkers of major depressive disorder: A case-control study. EBioMedicine. 2025;113:105569. doi: 10.1016/j.ebiom.2025.105569. - DOI - PMC - PubMed
-
- Picard M., McManus M.J., Gray J.D., Nasca C., Moffat C., Kopinski P.K., Seifert E.L., McEwen B.S., Wallace D.C. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl. Acad. Sci. USA. 2015;112:E6614–E6623. doi: 10.1073/pnas.1515733112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical