GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model
- PMID: 40806762
- PMCID: PMC12347635
- DOI: 10.3390/ijms26157634
GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model
Abstract
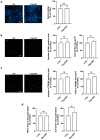
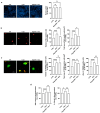
Neuroinflammation driven by microglial activation and α-synuclein (αSyn) aggregation is one of the central features driving Parkinson's disease (PD) pathogenesis. GM1 ganglioside's oligosaccharide moiety (OligoGM1) has shown neuroprotective potential in PD neuronal models, but its direct effects on inflammation remain poorly defined. This study investigated the ability of OligoGM1 to modulate microglial activation and αSyn handling in a human in vitro model. Human embryonic microglial (HMC3) cells were exposed to αSyn pre-formed fibrils (PFFs) in the presence or absence of OligoGM1. Microglial activation markers, intracellular αSyn accumulation, and cytokine release were assessed by immunofluorescence and ELISA. OligoGM1 had no effect on microglial morphology or cytokine release under basal conditions. Upon αSyn challenge, cells exhibited increased amounts of ionized calcium-binding adaptor molecule 1 (Iba1), triggered receptor expressed on myeloid cells 2 (TREM2), elevated αSyn accumulation, and secreted pro-inflammatory cytokines. OligoGM1 pre-treatment significantly reduced the number and area of Iba1(+) cells, the intracellular αSyn burden in TREM2(+) microglia, and the release of interleukin 6 (IL-6). OligoGM1 selectively attenuated αSyn-induced microglial activation and enhanced αSyn clearance without compromising basal immune function. These findings confirm and support the potential of OligoGM1 as a multitarget therapeutic candidate for PD that is capable of modulating glial reactivity and neuroinflammatory responses.
Keywords: GM1 oligosaccharide; Parkinson’s disease; microglia; neurodegeneration; α-synuclein.
Conflict of interest statement
Author Laura Rouvière, Alexandre Henriques, and Noelle Callizot were employed by the company Neuro-Sys. The remaining authors declare no conflicts of interest.
Figures
Similar articles
-
C18:0 GM3 ganglioside's efficacy in LPS-induced parkinsonism: neuroprotection, inflammatory inhibition and gliosis mitigation.Behav Brain Funct. 2025 Jul 26;21(1):25. doi: 10.1186/s12993-025-00289-8. Behav Brain Funct. 2025. PMID: 40713782 Free PMC article.
-
Parkinson's Paradox: Alpha-synuclein's Selective Strike on SNc Dopamine Neurons over VTA.bioRxiv [Preprint]. 2025 Apr 2:2025.03.24.644952. doi: 10.1101/2025.03.24.644952. bioRxiv. 2025. Update in: NPJ Parkinsons Dis. 2025 Jul 11;11(1):207. doi: 10.1038/s41531-025-01055-3. PMID: 40236072 Free PMC article. Updated. Preprint.
-
GM1 oligosaccharide-mediated rescue in GBA-linked Parkinson's disease via modulation of lysosomal and mitochondrial dysfunctions.Glycoconj J. 2025 Aug;42(3-4):159-171. doi: 10.1007/s10719-025-10185-y. Epub 2025 Jun 4. Glycoconj J. 2025. PMID: 40461737
-
Microglial immune memory in Parkinson's and Huntington's diseases: epigenetics, triggers, and therapies.Epigenomics. 2025 Aug;17(11):739-751. doi: 10.1080/17501911.2025.2518909. Epub 2025 Jul 1. Epigenomics. 2025. PMID: 40590831 Review.
-
Microglial activation as a hallmark of neuroinflammation in Alzheimer's disease.Metab Brain Dis. 2025 May 17;40(5):207. doi: 10.1007/s11011-025-01631-9. Metab Brain Dis. 2025. PMID: 40381069 Review.
References
-
- Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

