Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker
- PMID: 40806766
- PMCID: PMC12347763
- DOI: 10.3390/ijms26157638
Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker
Abstract
Tau protein is essential for the structural stability of neurons, particularly through its role in microtubule assembly and axonal transport. However, when abnormally hyperphosphorylated or cleaved, Tau can aggregate into insoluble forms that disrupt neuronal function, contributing to the pathogenesis of neurodegenerative diseases such as Alzheimer's disease (AD). Emerging evidence suggests that similar Tau-related alterations may occur in individuals with chronic exposure to psychoactive substances. This review compiles experimental, clinical, and postmortem findings that collectively indicate a substance-specific influence on Tau dynamics. Alcohol and opioids, for instance, promote Tau hyperphosphorylation and fragmentation through the activation of kinases such as GSK-3β and CDK5, as well as proteases like caspase-3, leading to neuroinflammation and microglial activation. Stimulants and dissociatives disrupt insulin signaling, increase oxidative stress, and impair endosomal trafficking, all of which can exacerbate Tau pathology. In contrast, cannabinoids and psychedelics may exert protective effects by modulating kinase activity, reducing inflammation, or enhancing neuroplasticity. Psychedelic compounds such as psilocybin and harmine have been demonstrated to decrease Tau phosphorylation and facilitate cognitive restoration in animal models. Although the molecular mechanisms differ across substances, Tau consistently emerges as a convergent target altered in substance-related cognitive disorders. Understanding these pathways may provide not only mechanistic insights into drug-induced neurotoxicity but also identify Tau as a valuable biomarker and potential therapeutic target for the prevention or treatment of cognitive decline associated with substance use.
Keywords: Tau protein; cognitive dysfunction; neurodegeneration; p-Tau; substance abuse.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


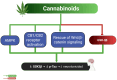

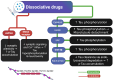
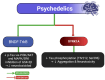
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Psychedelic-assisted therapy for treating anxiety, depression, and existential distress in people with life-threatening diseases.Cochrane Database Syst Rev. 2024 Sep 12;9(9):CD015383. doi: 10.1002/14651858.CD015383.pub2. Cochrane Database Syst Rev. 2024. PMID: 39260823
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- World Health Organization: Global Status Report on Alcohol and Health 2018. [(accessed on 23 June 2025)]. Available online: https://scholar.google.com/scholar_lookup?title=Global%20status%20report....
-
- World Drug Report 2021. [(accessed on 23 June 2025)]. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html.
-
- Substance Abuse and Mental Health Services Administration (US) Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration (US); Rockville, MD, USA: 2016. Substance Use Disorders. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

