Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance
- PMID: 40806771
- PMCID: PMC12347496
- DOI: 10.3390/ijms26157642
Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance
Abstract
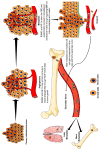
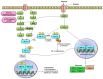
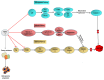
Runt-related transcription factor-2 (RUNX2) is an integral player in osteogenesis and is highly expressed in osteosarcoma. Emerging evidence suggests that aberrant RUNX2 expression is a key factor in osteosarcoma oncogenesis. Patients with advanced stages of osteosarcoma overexpressing RUNX2 are more likely to have high tumour grades, metastasis, and lower overall or progression-free survival rates. Thus, RUNX2 is considered a potential candidate for targeted therapy of osteosarcoma. Hypoxia-inducible factor-1α (HIF-1α) is a key transcription factor involved in the regulation of cellular reprogramming in response to hypoxia. Overexpression of HIF-1α decreases overall survival, disease-free survival, and chemotherapy response and promotes tumour stage and metastasis. Hence, our review focused on highlighting the intricate network between RUNX2 and HIF-1α, which support each other or may work synergistically to develop resistance to therapy and osteosarcoma progression. An in-depth understanding of these two important tumour progression markers is required. Therefore, this review focuses on the role of RUNX2 and HIF-1α in the alteration of the tumour microenvironment, which further promotes angiogenesis, metastasis, and resistance to therapy in osteosarcoma.
Keywords: HIF-1α; RUNX2; angiogenesis; metastasis; osteosarcoma; therapy resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Idoate M.Á., Aquerreta J.D., Lamo-Espinosa J.M., San-Julian M. A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations. Diagnostics. 2022;12:450. doi: 10.3390/diagnostics12020450. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

