The Link Between Human Alkyladenine DNA Glycosylase and Cancer Development
- PMID: 40806775
- PMCID: PMC12347255
- DOI: 10.3390/ijms26157647
The Link Between Human Alkyladenine DNA Glycosylase and Cancer Development
Abstract
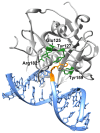
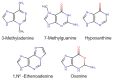
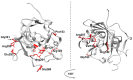
Alkyladenine DNA glycosylase (AAG) is a critical enzyme in the base excision repair (BER) pathway, responsible for removing a broad spectrum of alkylated DNA lesions. While AAG maintains genomic stability, dysregulated activity has been implicated in cancer development, drug resistance, and neurodegenerative diseases. This review synthesizes the current knowledge on AAG's structure, catalytic mechanism, and polymorphic variants, highlighting their potential roles in disease pathogenesis. A comprehensive bioinformatics analysis of over 370 AAG single-nucleotide polymorphisms (SNPs) is presented, identifying ~40% as high-risk variants likely to impair enzymatic function. Notably, 151 SNPs were predicted to be damaging by multiple algorithms, including substitutions at catalytic residues and non-conserved sites with unknown functional consequences. Analysis of cancer databases (COSMIC, cBioPortal, NCBI) revealed 93 tumor-associated AAG variants, with 18 classified as high-impact mutations. This work underscores the need for mechanistic studies of AAG variants using structural biology, cellular models, and clinical correlation analyses. Deciphering AAG's polymorphic landscape may unlock personalized strategies for cancer prevention and treatment.
Keywords: DNA repair; DNA repair coordination; alkyladenine DNA glycosylase; enzymatic activity; single-nucleotide polymorphism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Impact of DNA polymorphisms in key DNA base excision repair proteins on cancer risk.Hum Exp Toxicol. 2012 Oct;31(10):981-1005. doi: 10.1177/0960327112444476. Epub 2012 Sep 27. Hum Exp Toxicol. 2012. PMID: 23023028 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

