Aquaporins in the Capillaries of the Dura Mater of Pigs
- PMID: 40806781
- PMCID: PMC12347163
- DOI: 10.3390/ijms26157653
Aquaporins in the Capillaries of the Dura Mater of Pigs
Abstract
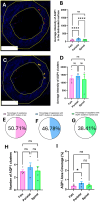
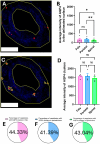
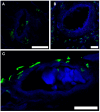
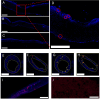
Dura mater plays a critical role in neurofluid homeostasis, yet comparative data on capillary network density and organization between cranial and spinal regions remain limited. This study addresses this gap by systematically analyzing capillary architecture and aquaporin (AQP) expression in porcine cranial (parietal, falx) and spinal dura mater. Immunofluorescence labeling and confocal microscopy were used to assess capillary density, spatial distribution, and AQP1/AQP4 expression patterns across over 1000 capillaries in these regions. Cranial dura exhibited a 3-4 times higher capillary density compared to spinal dura, with capillaries predominantly localized to meningeal-dural border cell interfaces in cranial regions and a more dispersed distribution in spinal dura. Both AQP1 and AQP4 were detected as discrete clusters within capillary walls, with higher expression in cranial compared to spinal dura. Lymphatic vessels (PDPN-positive) were also observed adjacent to capillaries, supporting a dual-system model for fluid and waste exchange. These findings highlight the dura's region-specific vascular specialization, with cranial regions favoring dense, structured capillary networks suited for active fluid exchange. This work establishes a foundation for investigating capillary-driven fluid dynamics in pathological states like subdural hematomas or hydrocephalus.
Keywords: Bulat–Klarica–Orešković hypothesis; aquaporin; capillary density; cerebrospinal fluid; cranial–spinal comparison; dura mater; lymphatic vessels; meningeal vascularization; porcine model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Heterogeneity in cranial dura mater at the microscale: An In-situ and ex-vivo structural and mechanical investigation of sulcus and gyrus Dura.Acta Biomater. 2025 Apr;196:222-232. doi: 10.1016/j.actbio.2025.02.053. Epub 2025 Feb 25. Acta Biomater. 2025. PMID: 40015354 Free PMC article.
-
The Connective Tissue Architecture of the Cavernous Sinus: An Anatomical Study and Unifying Conceptualization.World Neurosurg. 2025 Feb;194:123573. doi: 10.1016/j.wneu.2024.123573. Epub 2025 Jan 9. World Neurosurg. 2025. PMID: 39675667
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systematic review and meta-analysis of the biomechanical properties of the human dura mater applicable in computational human head models.Biomech Model Mechanobiol. 2022 Jun;21(3):755-770. doi: 10.1007/s10237-022-01566-5. Epub 2022 Mar 9. Biomech Model Mechanobiol. 2022. PMID: 35266061 Free PMC article.
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
References
-
- Ghannam J.Y., Al Kharazi K.A. StatPearls. StatPearls Publishing; Treasure Island, FL USA: 2024. Neuroanatomy, Cranial Meninges. - PubMed
-
- Hammady M. Histology of the Human Dura Mater; A Review Article. Egypt. J. Vet. Sci. 2023;54:907–916. doi: 10.21608/ejvs.2023.207485.1496. - DOI
-
- Morales-Avalos R., Soto-Domínguez A., García-Juárez J., Saucedo-Cardenas O., Bonilla-Galvan J.R., Cardenas-Serna M., Guzmán-López S., Elizondo-Omaña R.E. Characterization and Morphological Comparison of Human Dura Mater, Temporalis Fascia, and Pericranium for the Correct Selection of an Autograft in Duraplasty Procedures. Surg. Radiol. Anat. 2017;39:29–38. doi: 10.1007/s00276-016-1692-z. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

