A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications
- PMID: 40806812
- PMCID: PMC12347151
- DOI: 10.3390/jcm14155191
A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications
Abstract
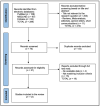
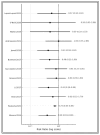
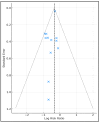
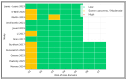
Background and Objectives: Postoperative pancreatic fistula and post-hepatectomy liver failure remain significant complications after HPB surgery; however, superficial surgical site infection (SSI) is the most frequent wound-related complication. Closed-incision negative-pressure wound therapy (ciNPWT) has been proposed to reduce superficial contamination, yet no liver-focused quantitative synthesis exists. We aimed to evaluate the effectiveness and safety of prophylactic ciNPWT after hepatopancreatobiliary (HPB) surgery. Methods: MEDLINE, Embase, and PubMed were searched from inception to 30 April 2025. Randomized and comparative observational studies that compared ciNPWT with conventional dressings after elective liver transplantation, hepatectomy, pancreatoduodenectomy, and liver resections were eligible. Two reviewers independently screened, extracted data, and assessed risk of bias (RoB-2/ROBINS-I). A random-effects Mantel-Haenszel model generated pooled risk ratios (RRs) for superficial SSI; secondary outcomes were reported descriptively. Results: Twelve studies (seven RCTs, five cohorts) encompassing 15,212 patients (3561 ciNPWT; 11,651 control) met the inclusion criteria. Device application lasted three to seven days in all trials. The pooled analysis demonstrated a 29% relative reduction in superficial SSI with ciNPWT (RR 0.71, 95% CI 0.63-0.79; p < 0.001) with negligible heterogeneity (I2 0%). Absolute risk reduction ranged from 0% to 13%, correlating positively with the baseline control-group SSI rate. Deep/organ-space SSI (RR 0.93, 95% CI 0.79-1.09) and 90-day mortality (RR 0.94, 95% CI 0.69-1.28) were unaffected. Seven studies documented a 1- to 3-day shorter median length of stay; only two reached statistical significance. Device-related adverse events were rare (one seroma, no skin necrosis). Conclusions: Prophylactic ciNPWT safely reduces superficial SSI after high-risk HPB surgery, with the greatest absolute benefit when baseline SSI risk exceeds ≈10%. Its influence on deep infection and mortality is negligible.
Keywords: closed-incision; hepatectomy; liver surgery; negative-pressure wound therapy; pancreatectomy; surgical site infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Interventions to prevent surgical site infection in adults undergoing cardiac surgery.Cochrane Database Syst Rev. 2024 Dec 2;12(12):CD013332. doi: 10.1002/14651858.CD013332.pub2. Cochrane Database Syst Rev. 2024. PMID: 39620424
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Topical antibiotics for preventing surgical site infection in wounds healing by primary intention.Cochrane Database Syst Rev. 2016 Nov 7;11(11):CD011426. doi: 10.1002/14651858.CD011426.pub2. Cochrane Database Syst Rev. 2016. PMID: 27819748 Free PMC article.
-
Intracavity lavage and wound irrigation for prevention of surgical site infection.Cochrane Database Syst Rev. 2017 Oct 30;10(10):CD012234. doi: 10.1002/14651858.CD012234.pub2. Cochrane Database Syst Rev. 2017. PMID: 29083473 Free PMC article.
References
-
- Gillespie B.M., Harbeck E., Rattray M., Liang R., Walker R., Latimer S., Thalib L., Andersson A.E., Griffin B., Ware R., et al. Worldwide incidence of surgical site infections in general surgical patients: A systematic review and meta-analysis of 488,594 patients. Int. J. Surg. 2021;95:106136. doi: 10.1016/j.ijsu.2021.106136. - DOI - PubMed
-
- Chambers L.E., Sheen A.J., Whitehead K.A. A systematic review on the incidence and risk factors of surgical site infections following hepatopancreatobiliary (HPB) surgery. AIMS Bioeng. 2022;9:123–144. doi: 10.3934/bioeng.2022010. - DOI
-
- Zaver V., Kankanalu P. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2025. [(accessed on 14 July 2025)]. Negative Pressure Wound Therapy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576388. - PubMed
-
- Groenen H., Jalalzadeh H., Buis D.R., Dreissen Y.E.M., Goosen J.H.M., Griekspoor M., Harmsen W.J., IJpma F.F.A., van der Laan M.J., Schaad R.R., et al. Incisional negative pressure wound therapy for the prevention of surgical site infection: An up-to-date meta-analysis and trial sequential analysis. EClinicalMedicine. 2023;62:102105. doi: 10.1016/j.eclinm.2023.102105. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

