Applications of Functional Near-Infrared Spectroscopy (fNIRS) in Monitoring Treatment Response in Psychiatry: A Scoping Review
- PMID: 40806818
- PMCID: PMC12347915
- DOI: 10.3390/jcm14155197
Applications of Functional Near-Infrared Spectroscopy (fNIRS) in Monitoring Treatment Response in Psychiatry: A Scoping Review
Abstract
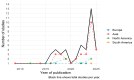
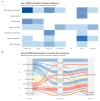
Background/Objective: Functional near-infrared spectroscopy (fNIRS) is a non-invasive neuroimaging technique with growing relevance in psychiatry. Its ability to measure cortical hemodynamics positions it as a potential tool for monitoring neurofunctional changes related to treatment. However, the specific features and level of consistency of its use in clinical psychiatric settings remain unclear. A scoping review was conducted under PRISMA-ScR guidelines to systematically map how fNIRS has been used in monitoring treatment response among individuals with psychiatric disorders. Methods: Forty-seven studies published between 2009 and 2025 were included based on predefined eligibility criteria. Data was extracted on publication trends, research design, sample characteristics, fNIRS paradigms, signal acquisition, preprocessing methods, and integration of clinical outcomes. Reported limitations and conflicts of interest were also analyzed. Results: The number of publications increased sharply after 2020, predominantly from Asia. Most studies used experimental designs, with 31.9% employing randomized controlled trials. Adults were the primary focus (93.6%), with verbal fluency tasks and DLPFC-targeted paradigms most common. Over half of the studies used high-density (>32-channel) systems. However, only 44.7% reported motion correction procedures, and 53.2% did not report activation direction. Clinical outcome linkage was explicitly stated in only 12.8% of studies. Conclusions: Despite growing clinical interest, with fNIRS showing promise as a non-invasive neuroimaging tool for monitoring psychiatric treatment response, the current evidence base is limited by methodological variability and inconsistent outcome integration. There is a rising need for the adoption of standardized protocols for both design and reporting. Future research should also include longitudinal studies and multimodal approaches to enhance validity and clinical relevance.
Keywords: brain activation scoping review; functional near-infrared spectroscopy; neuroimaging; psychiatric disorders; treatment monitoring; treatment response.
Conflict of interest statement
Of all the studies included, 45.07% (n = 32) reported small sample size as a limitation, 16.90% reported having no control/placebo/sham/healthy group (n = 12), 14.08% reported technical limitations (e.g., resolution issues, not enough NIRS channels; n = 10), and 14.08% reported having a short/no follow-up or no long-term measurements (n = 10, 10.94%). Limited generalizability/high heterogeneity (n = 6, 8.45%) was also mentioned, and one study [77] reported blinding issues. The summary of limitations reported is presented in Table 2.
The majority of studies had an explicit conflict of interest section (n = 43, 91.48%), while 8.52%% (n = 4) did not report any such statement. Among those that did report, the vast majority (77.6%, n = 38) explicitly stated that no conflicts of interest were present. A smaller portion (8.16%, n = 4) reported potential commercial or financial conflicts, typically involving research funding, personal fees, or equity holdings by one or more authors.
The authors declare no conflicts of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

