In Vitro Antimicrobial Activity of the Novel Antimicrobial Peptide OMN51 Against Multi-Drug-Resistant Pseudomonas aeruginosa Isolated from People with Cystic Fibrosis
- PMID: 40806829
- PMCID: PMC12347757
- DOI: 10.3390/jcm14155208
In Vitro Antimicrobial Activity of the Novel Antimicrobial Peptide OMN51 Against Multi-Drug-Resistant Pseudomonas aeruginosa Isolated from People with Cystic Fibrosis
Abstract
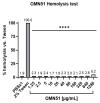
Background: People with cystic fibrosis (pwCF) frequently suffer from chronic lung infections, with Pseudomonas aeruginosa being the predominant pathogen contributing to disease progression and morbidity. The increasing prevalence of multi-drug-resistant (MDR) P. aeruginosa has diminished treatment options. Antimicrobial peptides (AMPs) have emerged as promising alternatives to conventional antibiotics due to their unique membrane-targeting mechanisms. OMN51, a novel bioengineered AMP derived from capitellacin, was evaluated for antimicrobial activity against P. aeruginosa in sputum samples from pwCF. This study aimed to compare the bactericidal effects of OMN51 with those of a range of conventional antibiotics known to have activity against P. aeruginosa clinical isolates derived from pwCF. Methods:P. aeruginosa clinical isolates were obtained from fifty-six unique sputum cultures of pwCF at a tertiary-university-affiliated hospital. Minimum inhibitory concentrations (MICs) of OMN51 and comparator antibiotics were determined using broth microdilution. Antimicrobial susceptibility was evaluated using the Kirby-Bauer disc diffusion method. Results: OMN51 demonstrated in vitro bactericidal activity across all P. aeruginosa isolates, including MDR strains. MIC values for OMN51 ranged from 4 to 16 µg/mL, with no observed resistance or cross-resistance. Comparative analysis revealed the superior efficacy of OMN51 compared with conventional antibiotics. Conclusions: OMN51 exhibits robust in vitro activity against MDR P. aeruginosa, supporting its candidacy as a therapeutic agent for MDR P. aeruginosa- associated infections. Further studies are warranted to assess pharmacokinetics and in vivo safety and efficacy. OMN51 represents a first-in-class, membrane-targeting therapeutic showing promise against MDR P. aeruginosa.
Keywords: OMN51; Pseudomonas aeruginosa; antibiotics; antimicrobial peptide; bactericidal; cystic fibrosis; in vitro; infections; multi-drug-resistant.
Conflict of interest statement
The following co-authors are employees of Omnix Medical and have shares or share options in Omnix Medical: M. Cohen-Kutner, E. Chass Maurice, N. Nur Maymon, S. Mandel, M. Oholy, R. Moses, M. Lavon, K. Kaufman, O. Mayost Lev-Ari, T. Shachar, and N. Bachnoff. OMN51 is covered by an international patent application published under application number WO 2024/003890 A1. The assignee is Omnix Medical, and the named inventors are N. Bachnoff, M. Cohen-Kutner, S. Mandel, and N. Nur Maymon.
Figures
Similar articles
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2024 May 3;5(5):CD009530. doi: 10.1002/14651858.CD009530.pub5. Cochrane Database Syst Rev. 2024. PMID: 38700027 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Combination antimicrobial susceptibility testing for acute exacerbations in chronic infection of Pseudomonas aeruginosa in cystic fibrosis.Cochrane Database Syst Rev. 2015 Nov 2;(11):CD006961. doi: 10.1002/14651858.CD006961.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Jun 19;6:CD006961. doi: 10.1002/14651858.CD006961.pub4. PMID: 26522473 Updated.
References
-
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

