Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis
- PMID: 40806845
- PMCID: PMC12347980
- DOI: 10.3390/jcm14155224
Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis
Abstract
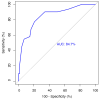
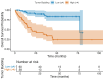
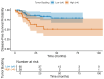
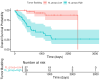
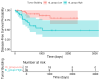
Background/Objectives: Tumor budding (TB)-clusters of one to five tumor cells at the invasive front-has emerged as a prognostic marker in various cancers. Its prognostic value in head and neck squamous cell carcinoma (HNSCC) is unclear. Methods: We retrospectively analyzed 98 HNSCC patients. The tumor buds were counted on hematoxylin-eosin-stained sections as per the 2016 International Tumor Budding Consensus Conference (ITBCC) guidelines. An optimal cutoff was determined by ROC analysis using excisional lymph nodes and five-year overall survival (OS) as the endpoint, stratifying patients into low- (≤4 buds) and high-risk (>4 buds) groups. The associations with clinicopathological features, OS, and disease-free survival (DFS) were assessed using Kaplan-Meier curves and Cox regression. Results: Among the 98 patients (median follow-up 58 months, range 18-108), 32 (32.7%) died. The optimal TB cutoff was 4.5 (AUC 0.85, 95% CI 0.76-0.93). High TB was associated with poorer five-year OS (26.4% vs. 85.3%). Multivariate Cox regression identified TB and extranodal extension as independent predictors of OS (TB HR: 3.4, 95% CI 1.3-9.2, p = 0.013). In the laryngeal cancer subgroup, TB was associated with worse survival in the univariate analysis (HR 7.5, 95% CI 1.6-35.6, p = 0.011), though this was not significant in the multivariate modeling. High TB independently predicted neck lymph node metastasis (multivariate OR 4.9, 95% CI 1.2-20.5, p = 0.029), which was present in 65.8% of the high-TB vs. 31.7% of the low-TB patients. High TB correlated with advanced AJCC stage and lymphovascular invasion. No clinicopathological factors, including TB, independently predicted DFS, in either the full cohort or the laryngeal subgroup. Conclusions: High tumor budding denotes an aggressive HNSCC phenotype and may guide decisions on elective neck dissection. Its assessment is simple, cost-effective, and potentially valuable for routine pathology, pending external validation.
Keywords: head and neck cancer; head and neck squamous cell carcinoma; neck lymph node metastasis; overall survival; prognosis; tumor budding.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Risk Stratification for Extranodal Extension in Head and Neck Cancers-Implication for Treatment Intensification.Head Neck. 2025 Aug;47(8):2270-2279. doi: 10.1002/hed.28144. Epub 2025 Mar 28. Head Neck. 2025. PMID: 40151024
-
Potential predictor of prognosis in breast carcinoma: Tumor budding.Indian J Pathol Microbiol. 2025 Apr 1;68(2):317-323. doi: 10.4103/ijpm.ijpm_300_24. Epub 2024 Sep 14. Indian J Pathol Microbiol. 2025. PMID: 40682770
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Tumor Budding as an Independent Prognostic Histopathological Marker in Oral Squamous Cell Carcinoma - An Indian Tertiary Care Center Experience.Turk Patoloji Derg. 2025;41(2):31-41. doi: 10.5146/tjpath.2025.13761. Turk Patoloji Derg. 2025. PMID: 40091314 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Almangush A., Bello I.O., Keski-Säntti H., Mäkinen L.K., Kauppila J.H., Pukkila M., Hagström J., Laranne J., Tommola S., Nieminen O., et al. Depth of invasion, tumor budding, and worst pattern of invasion: Prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–818. doi: 10.1002/hed.23380. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

