Possession of Injectable Epinephrine Among Children with Parent-Reported Food Allergies in Saudi Arabia
- PMID: 40806896
- PMCID: PMC12347483
- DOI: 10.3390/jcm14155274
Possession of Injectable Epinephrine Among Children with Parent-Reported Food Allergies in Saudi Arabia
Abstract
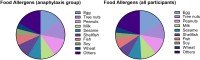
Background/Objectives: A food allergy (FA) is an immune-mediated hypersensitivity reaction to specific food. FA reactions vary from mild to life-threatening anaphylaxis. Despite the effectiveness of epinephrine auto-injectors (EAIs), barriers such as lack of knowledge, limited access, and fear of needles hinder their use. This study explores EAI possession among children with parent-reported food allergies in Saudi Arabia. Methods: A cross-sectional study conducted from October 2023 to February 2024 included 296 parents of children with reported food allergies under the age of 18. Data were collected through a validated self-administered questionnaire. Results: Among 2102 respondents, 296 (14.1%) reported having a child with a food allergy. Most respondents were female (70%), with asthma being the most common comorbidity (26%). Common allergens included eggs, tree nuts, peanuts, milk, and sesame. Only 23.3% of children had an EAI. Higher EAI possession was associated with parental education, maternal allergy history, and access to specialist care. Conclusions: EAI possession among Saudi children with food allergies is suboptimal. Targeted educational interventions, increased access to allergists, and comprehensive management plans are essential to improve preparedness for anaphylaxis.
Keywords: Saudi Arabia; epinephrine; epinephrine auto-injector; food allergy; parental education.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Allergen-specific oral immunotherapy for peanut allergy.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009014. doi: 10.1002/14651858.CD009014.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972130 Free PMC article.
-
Self-reported diagnoses of dietary allergens and fecundability in a North American cohort.Hum Reprod. 2025 Mar 1;40(3):553-560. doi: 10.1093/humrep/deae277. Hum Reprod. 2025. PMID: 39719047
-
A systematic review and cost-effectiveness analysis of specialist services and adrenaline auto-injectors in anaphylaxis.Health Technol Assess. 2013 Apr;17(17):1-117, v-vi. doi: 10.3310/hta17170. Health Technol Assess. 2013. PMID: 23618619 Free PMC article.
-
Food Allergy in Children in China.Clin Exp Allergy. 2025 Aug;55(8):634-647. doi: 10.1111/cea.14596. Epub 2024 Dec 6. Clin Exp Allergy. 2025. PMID: 39641430 Free PMC article. Review.
References
-
- Calvani M., Anania C., Caffarelli C., Martelli A., Miraglia Del Giudice M., Cravidi C., Duse M., Manti S., Tosca M.A., Cardinale F., et al. Food allergy: An updated review on pathogenesis, diagnosis, prevention and management. Acta Biomed. 2020;91:e2020012. doi: 10.23750/abm.v91i11-S.10316. - DOI - PMC - PubMed
-
- Lieberman J., Sublett J., Ali Y., Haselkorn T., Damle V., Chidambaram A., Rosen K., Mahr T. Increased incidence and prevalence of peanut allergy in children and adolescents in the United States. Ann. Allergy Asthma Immunol. 2018;121:S13. doi: 10.1016/j.anai.2018.09.039. - DOI
-
- Park J., Ahn S., Sicherer S. Prevalence of allergy to multiple versus single foods in a pediatric food allergy referral practice. J. Allergy Clin. Immunol. 2010;125:AB216. doi: 10.1016/j.jaci.2009.12.843. - DOI
LinkOut - more resources
Full Text Sources