Efficacy and Safety of a Balanced Gelatine Solution for Fluid Resuscitation in Sepsis: A Prospective, Randomised, Controlled, Double-Blind Trial-GENIUS Trial
- PMID: 40806945
- PMCID: PMC12346933
- DOI: 10.3390/jcm14155323
Efficacy and Safety of a Balanced Gelatine Solution for Fluid Resuscitation in Sepsis: A Prospective, Randomised, Controlled, Double-Blind Trial-GENIUS Trial
Abstract
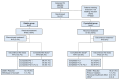
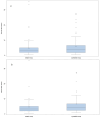
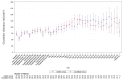
Background/Objective: Sepsis is a leading cause of death in noncoronary intensive care units (ICUs). Fluids for intravascular resuscitation include crystalloids and colloids. There is extensive clinical evidence on colloid use, but large trials comparing gelatine with crystalloid regimens in ICU and septic patients are lacking. This study aimed to determine whether early, protocol-driven volume resuscitation using a gelatine-based regimen achieves hemodynamic stability (HDS) more rapidly than a crystalloid-based regimen in septic patients. Methods: This prospective, controlled, randomised, double-blind, multinational phase IV study compared two parallel groups of septic patients receiving a gelatine-based regimen (Gelaspan® 4% and Sterofundin® ISO, B. Braun Melsungen AG each, at a 1:1 ratio) or a crystalloid regimen (Sterofundin® ISO). Primary endpoint was time to first HDS within 48 h after randomisation. Secondary endpoints included fluid overload, fluid balance, and patient outcomes. Results: 167 patients were randomised. HDS was achieved after 4.7 h in the gelatine group and after 5.8 h in the crystalloid group (p = 0.3716). The gelatine group had a more favourable fluid balance at 24 h (medians: 3463.00 mL vs. 4164.00 mL; p = 0.0395) and less fluid overload (medians: 4296.05 vs. 5218.75%; p = 0.0217). No differences were observed in serious adverse events or mortality. Conclusions: The study provided clinical evidence of balanced gelatine solution for volume resuscitation in septic patients, although it was terminated prematurely. The early and protocol-based administration of gelatine was safe and effective in the enrolled patient population. Time to HDS was not different between groups but the gelatine-based regimen led to better fluid balance and less fluid overload.
Keywords: crystalloid; fluid balance; fluid resuscitation; gelatine; sepsis.
Conflict of interest statement
D.F. received lecture and advisory fees as well as grants and scientific support from Astra Zeneca, Baxter, B. Braun Melsungen AG, Cytosorb, CSL Behring, Haemonetics, IL Werfen, LFB France, Mitsubishi Pharma, and Octapharm. G.M. received restricted research grants and consultancy fees from B. Braun Melsungen AG, 4Teen4, and Adrenomed AG outside of the submitted work. J.E. received financial support for traveling to investigator meetings of the GENIUS trial from B. Braun Melsungen AG. The Department of Anaesthesiology, Intensive Care Medicine & Pain Therapy of the University Hospital Frankfurt, Goethe University received support from B. Braun Melsungen AG, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt‘s Patient Blood Management program. K.Z. has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring, Masimo, Pharmacosmos, Boston Scientific, Salus, iSEP, Edwards, and GE Healthcare. K.Z. is the Principal Investigator of the EU-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of COVID-19 patients and smart decision-making in Intensive Care Units) and Horizon Europe 2021 project COVend (Biomarker and AI-supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID-19). K.Z. leads, as CEO, the Christoph Lohfert Foundation as well as the Health, Patient Safety & PBM Foundation. T.P.S. received travel grants and lecture or consultancy fees from B. Braun Melsungen AG, Sphingotec GmbH, Hennigsdorf, Germany. The other authors declare no conflicts of interest.
Figures
Similar articles
-
Efficacy and safety of early target-controlled plasma volume replacement with a balanced gelatine solution versus a balanced electrolyte solution in patients with severe sepsis/septic shock: study protocol, design, and rationale of a prospective, randomized, controlled, double-blind, multicentric, international clinical trial : GENIUS-Gelatine use in ICU and sepsis.Trials. 2021 Jun 2;22(1):376. doi: 10.1186/s13063-021-05311-8. Trials. 2021. PMID: 34078421 Free PMC article.
-
Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality.BMJ. 2014 Jul 22;349:g4561. doi: 10.1136/bmj.g4561. BMJ. 2014. PMID: 25099709 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Colloids versus crystalloids for fluid resuscitation in critically ill patients.Cochrane Database Syst Rev. 2004 Oct 18;(4):CD000567. doi: 10.1002/14651858.CD000567.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD000567. doi: 10.1002/14651858.CD000567.pub3. PMID: 15495001 Updated.
-
Colloids versus crystalloids for fluid resuscitation in critically ill patients.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD000567. doi: 10.1002/14651858.CD000567.pub6. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Aug 03;8:CD000567. doi: 10.1002/14651858.CD000567.pub7. PMID: 23450531 Updated.
References
LinkOut - more resources
Full Text Sources

