Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism
- PMID: 40807023
- PMCID: PMC12347908
- DOI: 10.3390/jcm14155403
Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism
Abstract
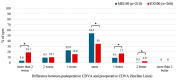
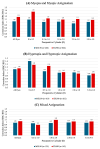
Background/Objectives: Although both the MEL90 (Carl Zeiss Meditec AG, Jena, Germany) and WaveLight EX500 (Alcon Laboratories, Inc., Fort Worth, TX, USA) are two widely used excimer lasers, comparisons between the two remain limited. This study evaluates visual and refractive outcomes from the U.S. Food and Drug Administration (FDA) premarket approval trials of these platforms in the treatment of myopia with and without astigmatism, hyperopia with and without astigmatism, and mixed astigmatism. Methods: Clinical outcomes from FDA premarket approval trials were compared between the recently approved MEL90 and the WaveLight (now termed EX500) excimer lasers. Results: A total of 714 eyes (358 patients) from MEL90 and 1353 eyes (706 patients) from EX500 were analyzed up to 6 months postoperatively. In the hyperopia/hyperopic astigmatism cohort, the EX500 demonstrated greater efficacy relative to MEL90, with more eyes achieving a postoperative uncorrected distance visual acuity (UDVA) of 20/20 or better (48.6% vs. 68.7%, respectively; p < 0.001). In both the MEL90 and EX500, at least 85% of eyes with myopia/myopic astigmatism and 68% with mixed astigmatism achieved a postoperative UDVA of 20/20 or better. For all refractive cohorts, more than 95% of eyes achieved a UDVA of 20/40 or better at 6 months (all p > 0.05). The EX500 was more likely to demonstrate an improvement of more than two lines of UDVA compared to baseline CDVA (all p < 0.05). In contrast, the MEL90 showed greater predictability of spherical equivalent within ±0.50 D and ±1.00 D for the hyperopia/hyperopic astigmatism cohort (both p = 0.007), as well as within ±0.50 D for the myopia/myopic astigmatism cohort (p < 0.001). Postoperatively, both platforms were associated with decreased glare and halos, although findings were variable in the EX500 mixed astigmatism cohort. Conclusions: Both excimer lasers demonstrated safe and effective outcomes that exceed the threshold set by the FDA.
Keywords: EX500; LASIK; MEL90; WaveLight; excimer laser; predictability; refractive surgery; safety.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Clinical Evaluation of the Stationary Scanning-Spot EX500 Excimer Laser System for Hyperopic LASIK in Patients With Hyperopia With and Without Astigmatism.J Refract Surg. 2025 Jul;41(7):e655-e661. doi: 10.3928/1081597X-20250520-04. Epub 2025 Jul 1. J Refract Surg. 2025. PMID: 40626427 Clinical Trial.
-
Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD007679. doi: 10.1002/14651858.CD007679.pub4. Cochrane Database Syst Rev. 2014. PMID: 24937100 Free PMC article.
-
Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia.Cochrane Database Syst Rev. 2012 Jan 18;1:CD007679. doi: 10.1002/14651858.CD007679.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 17;(6):CD007679. doi: 10.1002/14651858.CD007679.pub4. PMID: 22258972 Updated.
-
Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia.Cochrane Database Syst Rev. 2010 May 12;(5):CD007679. doi: 10.1002/14651858.CD007679.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2012 Jan 18;1:CD007679. doi: 10.1002/14651858.CD007679.pub3. PMID: 20464757 Updated.
-
Laser-assisted subepithelial keratectomy (LASEK) versus laser-assisted in-situ keratomileusis (LASIK) for correcting myopia.Cochrane Database Syst Rev. 2017 Feb 15;2(2):CD011080. doi: 10.1002/14651858.CD011080.pub2. Cochrane Database Syst Rev. 2017. PMID: 28197998 Free PMC article.
References
-
- Shehata A.M. Femtosecond Lasik Versus SMILE for Management of High Myopic Astigmatism: Six Months Outcome. Med. J. Cairo Univ. 2023;91:849–856. doi: 10.21608/mjcu.2023.318547. - DOI
-
- FDA, Center for Devices and Radiological Health List of FDA-Approved Lasers for LASIK. FDA. Published Online 3 November 2018. [(accessed on 19 June 2025)]; Available online: https://www.fda.gov/medical-devices/lasik/list-fda-approved-lasers-lasik.
LinkOut - more resources
Full Text Sources

