Comparative Analysis of the Long-Term Real-World Efficacy of Interleukin-17 Inhibitors in a Cohort of Patients with Moderate-to-Severe Psoriasis Treated in Poland
- PMID: 40807042
- PMCID: PMC12347414
- DOI: 10.3390/jcm14155421
Comparative Analysis of the Long-Term Real-World Efficacy of Interleukin-17 Inhibitors in a Cohort of Patients with Moderate-to-Severe Psoriasis Treated in Poland
Abstract
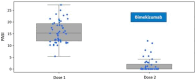
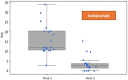
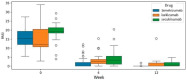
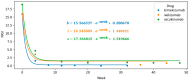
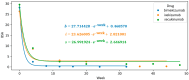
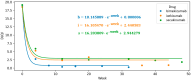
Background: Bimekizumab, secukinumab, and ixekizumab are IL-17-targeting biologics approved for the treatment of moderate-to-severe plaque psoriasis. While secukinumab and ixekizumab selectively inhibit IL-17A, bimekizumab targets both IL-17A and IL-17F, potentially providing greater anti-inflammatory efficacy. This study aimed to compare the real-world effectiveness, safety, and tolerability of these agents in a Polish dermatology center between 2019 and 2024. Methods: We conducted a retrospective analysis of 98 patients meeting at least one of the following criteria: PASI ≥ 10, BSA ≥ 10, DLQI ≥ 10, or involvement of special areas with inadequate response or contraindications to ≥2 systemic therapies. Patients with prior exposure only to IL-17 inhibitors were excluded. PASI, BSA, and DLQI scores were recorded at baseline, week 4, and week 12. Due to differences in dosing schedules, outcomes were aligned using standardized timepoints and exponential modeling of continuous response trajectories. Mixed-effects ANOVA was used to assess the influence of baseline factors (age, BMI, PsA status) on treatment outcomes. Adverse events were documented at each monthly follow-up visit. Results: Bimekizumab showed the greatest effect size for PASI reduction (Hedges' g = 3.662), followed by secukinumab (2.813) and ixekizumab (1.986). Exponential modeling revealed a steeper response trajectory with bimekizumab (intercept = 0.289), suggesting a more rapid PASI improvement. The efficacy of bimekizumab was particularly notable in patients who were previously treated with IL-23 inhibitors. All three agents demonstrated favorable safety profiles, with no serious adverse events or discontinuations. The most frequent adverse events were mild and included upper respiratory tract infections and oral candidiasis. Conclusions: This real-world analysis confirmed that IL-17 inhibitors effectively improved PASI, BSA, and DLQI scores in moderate-to-severe psoriasis. Bimekizumab demonstrated the most rapid early improvements and a higher modeled likelihood of complete clearance, without significant differences at week 12. All agents were well tolerated, underscoring the need for further individualized, large-scale studies.
Keywords: Interleukin Inhibitors; bimekizumab; interleukin-17; ixekizumab; psoriasis; secukinumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2025 Aug 6;8(8):CD011535. doi: 10.1002/14651858.CD011535.pub7. Cochrane Database Syst Rev. 2025. PMID: 40767824
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2022 May 23;5(5):CD011535. doi: 10.1002/14651858.CD011535.pub5. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Jul 12;7:CD011535. doi: 10.1002/14651858.CD011535.pub6. PMID: 35603936 Free PMC article. Updated.
-
Bimekizumab efficacy and safety through 3 years in patients with moderate-to-severe plaque psoriasis: long-term results from the BE RADIANT phase IIIb trial open-label extension period.Br J Dermatol. 2025 Jun 20;193(1):44-55. doi: 10.1093/bjd/ljaf032. Br J Dermatol. 2025. PMID: 39862230 Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

