Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study
- PMID: 40807093
- PMCID: PMC12346947
- DOI: 10.3390/jcm14155473
Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study
Abstract
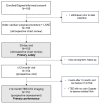
Background: Cardiac surgery patients with pre- or post-operative atrial fibrillation are at an increased risk for thromboembolic stroke, often due left atrial appendage (LAA) thrombus. Surgical LAA exclusion (LAAE) can be performed and must be complete to avoid increased thrombus formation. Methods: This prospective, multi-center, post-market study (NCT05101993) evaluated the long-term safety and performance of the epicardial V-shape AtriClip device. Patients ≥18 years who had received V-shape AtriClip devices during non-emergent cardiac surgery consented to a prospective 12-month follow-up visit and LAA imaging. The primary performance was LAAE without residual left atrium-LAA communication, assessed by imaging at the last follow-up visit. The primary safety was device- or implant procedure-related serious adverse events (SAEs) (death, major bleeding, surgical site infection, pericardial effusion requiring intervention, myocardial infarction) within 30 days. Results: Of 155 patients from 11 U.S. centers, 151 patients had evaluable imaging. Complete LAAE was obtained in all patients. Primary performance in the intent-to-treat population was met, with 97% (95% CI 93.52%, 99.29%; p = 0.0001) complete LAAE. Primary safety was met, with 100% (95% CI 97.75%, 100%; p < 0.0001) of patients free from pre-defined SAEs within 30 days. One device-related SAE was reported, which resolved intraprocedurally. Conclusions: AtriClip V-Clip showed safe and successful LAAE through 12 months of follow-up.
Keywords: AtriClip; cardiac surgery; left atrial appendage.
Conflict of interest statement
Dr. Zias: AtriCure: consultant; Dr. Gerdisch: AtriCure: consultant, research; Artivion: consultant, research; Arthrex: consultant; Abbott: consultant; Corvivo: consultant, research; DASI Simulations: consultant, research; PleuraFlow: consultant; Dr. Moront: Medtronic Heart Valves: consultant and trainer; AtriCure: consultant and trainer; Haemonetics: lecturer; Dr. Johnson: AtriCure: speaker; Dr. El-Eshmawi: None; Dr. Bhatia: Baxter: consultant; Dr. Kasten: AtriCure: consultant; Dr. Saum: AtriCure: proctor, consultant; Dr. Takayama: Artivion: consultant; Edwards: consultant; Dr. Singh: None; Dr. Damiano: Edwards: consultant; Medtronic: consultant; AtriCure: speaker, research.
Figures

References
-
- Writing Committee Members. Joglar J.A., Chung M.K., Armbruster A.L., Benjamin E.J., Chyou J.Y., Cronin E.M., Deswal A., Eckhardt L.L., Goldberger Z.D., et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023;83:109–279. doi: 10.1016/j.jacc.2023.08.017. - DOI - PMC - PubMed
-
- Tzeis S., Gerstenfeld E.P., Kalman J., Saad E., Shamloo A.S., Andrade J.G., Barbhaiya C.R., Baykaner T., Boveda S., Calkins H., et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2024;67:921–1072. doi: 10.1007/s10840-024-01771-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

