Does the Timing of Response Impact the Outcome of Relapsed/Refractory Acute Myeloid Leukemia Treated with Venetoclax in Combination with Hypomethylating Agents? A Proof of Concept from a Monocentric Observational Study
- PMID: 40807220
- PMCID: PMC12347298
- DOI: 10.3390/jcm14155586
Does the Timing of Response Impact the Outcome of Relapsed/Refractory Acute Myeloid Leukemia Treated with Venetoclax in Combination with Hypomethylating Agents? A Proof of Concept from a Monocentric Observational Study
Abstract
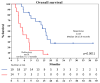
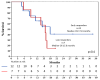
Background: Relapsed/refractory acute myeloid leukemia (R/R AML) remains a therapeutic challenge due to disease heterogeneity, resistance mechanisms, and poor tolerability to intensive regimens. Venetoclax (VEN), a BCL-2 inhibitor, has shown promise in combination with hypomethylating agents (HMAs), but data on response timing in the R/R setting are limited. The aim of this study was to assess the efficacy, safety, and kinetics of response to HMA-VEN therapy in a real-world cohort of R/R AML patients, with particular focus on early versus late responders. Methods: This prospective single-center study included 33 adult patients with R/R AML treated with VEN plus either azacitidine (AZA) or decitabine (DEC) from 2018 to 2021. The primary endpoint was the composite complete remission (cCR) rate and the rate of early and late response, respectively, occurring within two cycles of therapy or later; secondary endpoints included overall survival (OS), relapse-free survival (RFS), time to relapse (TTR), and safety. Results: The cCR was 58%, with complete remission (CR) or CR with incomplete recovery (CRi) achieved in 52% of patients. Median OS was 9 months. No significant differences in OS or TTR were observed between early (≤2 cycles) and late (>2 cycles) responders. Eight responders (42%) underwent allogeneic hematopoietic stem cell transplantation (HSCT), with comparable transplant rates in both groups of responders. Toxicity was manageable. Grade 3-4 neutropenia occurred in all patients, and febrile neutropenia occurred in 44% of patients. An Eastern Cooperative Oncology Group (ECOG) score >2 was associated with inferior response and shorter treatment duration. Conclusions: HMA-VEN therapy is effective and safe in R/R AML, including for patients with delayed responses. The absence of a prognostic disadvantage for late responders supports flexible treatment schedules and suggests that the continuation of therapy may be beneficial even without early blast clearance. Tailored approaches based on performance status and comorbidities are warranted, and future studies should incorporate minimal residual disease (MRD)-based monitoring to refine response assessment.
Keywords: Acute Myeloid Leukemia; cytopenia; early response; hypomethylating Agents; late response; relapsed/refractory AML; response rate; treatment related-toxicity; venetoclax; washout period.
Conflict of interest statement
C.V. declares being a member of the advisory board for AbbVie, Astellas, Jazz, BMS, and Otsuka. The remaining authors declare no relevant conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Efficacy and prognostic analysis of venetoclax combined with hypomethylating agents for induction therapy in acute myeloid leukemia: a multi-center real-world study on indication-specific stratification, molecular markers, and hematologic toxicities.Cancer Cell Int. 2025 Jul 1;25(1):241. doi: 10.1186/s12935-025-03858-z. Cancer Cell Int. 2025. PMID: 40598544 Free PMC article.
-
Oral decitabine and cedazuridine plus venetoclax for older or unfit patients with acute myeloid leukaemia: a phase 2 study.Lancet Haematol. 2024 Apr;11(4):e276-e286. doi: 10.1016/S2352-3026(24)00033-4. Epub 2024 Mar 4. Lancet Haematol. 2024. PMID: 38452788 Free PMC article. Clinical Trial.
-
A systematic overview of chemotherapy effects in acute myeloid leukaemia.Acta Oncol. 2001;40(2-3):231-52. doi: 10.1080/02841860151116321. Acta Oncol. 2001. PMID: 11441935
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Vecchio V., Duminuco A., Leotta S., Mauro E., Maugeri C., Parisi M., Fiumara P.F., Di Raimondo F., Palumbo G.A., Gozzo L., et al. Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort. J. Clin. Med. 2025;14:5110. doi: 10.3390/jcm14145110. - DOI - PMC - PubMed
-
- Ferrara F., Barosi G., Venditti A., Angelucci E., Gobbi M., Pane F., Tosi P., Zinzani P., Tura S. Consensus-Based Definition of Unfitness to Intensive and Non-Intensive Chemotherapy in Acute Myeloid Leukemia: A Project of SIE, SIES and GITMO Group on a New Tool for Therapy Decision Making. Leukemia. 2013;27:997–999. doi: 10.1038/leu.2012.303. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous