Synthesis and Electronic Properties of Novel Donor-π-Acceptor-Type Functional Dyes with a Carbonyl-Bridged Bithiophene π-Spacer
- PMID: 40807260
- PMCID: PMC12348573
- DOI: 10.3390/molecules30153084
Synthesis and Electronic Properties of Novel Donor-π-Acceptor-Type Functional Dyes with a Carbonyl-Bridged Bithiophene π-Spacer
Abstract
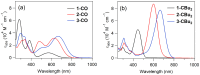
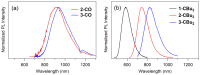
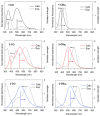
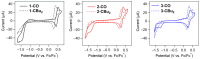
In this study, we synthesized novel donor-π-acceptor (D-π-A) functional dyes bearing a carbonyl-bridged bithiophene as a π-conjugated spacer and evaluated the absorption and fluorescence properties as well as the photostability. The developed dyes 1-CO-3-CO possess an N,N-diphenylaminophenyl electron donor unit and an electron acceptor unit such as a formyl group (1-CO), an (N,N-diethylthiobarbituryl)methylene moiety (2-CO), or a (3-dicyanomethylidene-1-indanon-2-yl)methylene moiety (3-CO). The absorption spectra of 1-CO-3-CO in dichloromethane at room temperature showed absorption maxima at 569 nm, 631 nm, and 667 nm, respectively, and the stronger acceptors in 2-CO and 3-CO led to enhancement of the ICT character. In addition, 2-CO and 3-CO had a second absorption band in the visible region, showing panchromatic absorption properties. Electrochemical analyses of the developed dyes revealed that the carbonyl bridging group in the π-spacer contributes to stabilization of the frontier orbitals such as the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO, respectively), in comparison with the referential dyes bearing a dibutylmethylene-bridged bithiophene spacer, 1-CBu2-3-CBu2. The HOMO/LUMO stabilization brought about high photostability in the doped poly(methyl methacrylate) film.
Keywords: donor–π–acceptor-type dye; frontier orbital; intramolecular charge transfer; panchromatic absorption; photostability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Molecular Design and DFT Analysis of High-performance Dyes Based on Pyrene with Different Donor Parts and Their Optoelectronic Applications.J Fluoresc. 2025 Jul;35(7):5487-5499. doi: 10.1007/s10895-024-03936-x. Epub 2024 Sep 14. J Fluoresc. 2025. PMID: 39271601
-
Synthesis and Physical Properties of [n]Cycloparaphenylene Ketone (n = 6, 7, 8, and 10).Angew Chem Int Ed Engl. 2025 Aug 11;64(33):e202509754. doi: 10.1002/anie.202509754. Epub 2025 Jun 29. Angew Chem Int Ed Engl. 2025. PMID: 40498084
-
Phenothiazine Polymers as Versatile Electrode Materials for Next-Generation Batteries.Acc Mater Res. 2025 May 19;6(6):754-764. doi: 10.1021/accountsmr.5c00053. eCollection 2025 Jun 27. Acc Mater Res. 2025. PMID: 40611976 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Co-formulated abacavir-lamivudine-zidovudine for initial treatment of HIV infection and AIDS.Cochrane Database Syst Rev. 2013 Mar 28;2013(3):CD005481. doi: 10.1002/14651858.CD005481.pub3. Cochrane Database Syst Rev. 2013. PMID: 23543540 Free PMC article.
References
-
- Bures F. Fundamental Aspects of Property Tuning in Push–Pull Molecules. RSC Adv. 2014;4:58826. doi: 10.1039/C4RA11264D. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

