Neuroprotective Evaluation of Murraya Carbazoles: In Vitro and Docking Insights into Their Anti-AChE and Anti-Aβ Activities
- PMID: 40807313
- PMCID: PMC12348157
- DOI: 10.3390/molecules30153138
Neuroprotective Evaluation of Murraya Carbazoles: In Vitro and Docking Insights into Their Anti-AChE and Anti-Aβ Activities
Abstract
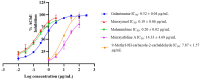
The present study investigated the neuroprotective potential of the Murraya carbazole derivatives murrayanol, mahanimbine, murrayafoline A, and 9-methyl-9H-carbazole-2-carbaldehyde using in silico and in vitro assays. The pharmacokinetic properties and potential toxicity (ADME/T) of the carbazole derivatives were assessed to evaluate their prospects as up-and-coming drug candidates. Molecular docking was used to investigate the interactions of the compounds with Aβ (PDB: 1IYT, 2BEG, and 8EZE) and AChE receptors (PDB: 4EY7 and 1C2B). The results from the in vitro assays were used to validate and support the findings from the in silico assays. The compounds demonstrated significant inhibition of acetylcholinesterase (AChE), a key target in neurodegenerative disorders. Murrayanol and mahanimbine presented superior inhibitory activity (IC50 ~0.2 μg/mL), outperforming the reference drug, galantamine. The inhibition mechanisms were competitive (murrayanol, murrayafoline A, and 9-methyl-9H-carbazole-2-carbaldehyde) and non-competitive (mahanimbine), supported by low Ki values and strong docking affinities. The compounds also proved effective in reducing Aβ fibrillization (murrayanol: 40.83 ± 0.30%; murrayafoline A: 33.60 ± 0.55%, mahanimbine: 27.68 ± 2.71%). These findings highlight Murraya carbazoles as promising scaffolds for multifunctional agents in AD therapy. Further optimization and mechanistic studies are warranted to advance their development into clinically relevant neuroprotective agents.
Keywords: ADME/T properties; acetylcholinesterase; anti-Aβ fibrilization; carbazole; molecular docking; neuroprotection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
In vitro and in vivo evaluation of 1,4-bis-benzylpiperazine-2-carboxylic acid derivatives as potential multi-target directed ligands (MTDLs) anti-Alzheimer's agents.Bioorg Chem. 2025 Aug;163:108750. doi: 10.1016/j.bioorg.2025.108750. Epub 2025 Jul 14. Bioorg Chem. 2025. PMID: 40700929
-
ML-based prediction to experimental validation: Development of dihydroquinazoline based multi-potent ligands as anti-Alzheimer's agents.Comput Biol Med. 2025 Sep;196(Pt A):110762. doi: 10.1016/j.compbiomed.2025.110762. Epub 2025 Jul 14. Comput Biol Med. 2025. PMID: 40664126
-
Design, synthesis, and biological evaluation of caffeic acid-based novel multifunctional molecules for the management of Alzheimer's disease.Eur J Med Chem. 2025 Oct 15;296:117831. doi: 10.1016/j.ejmech.2025.117831. Epub 2025 Jun 10. Eur J Med Chem. 2025. PMID: 40578253
-
Therapeutic potential of morpholine-based compounds in neurodegenerative diseases: SAR insights and analysis.Future Med Chem. 2025 Jun;17(12):1439-1455. doi: 10.1080/17568919.2025.2515812. Epub 2025 Jun 8. Future Med Chem. 2025. PMID: 40485213 Review.
-
Targeting Acetylcholinesterase with Oxytocin: A New Avenue in Alzheimer's Disease Therapeutics.ACS Chem Neurosci. 2025 Jul 2;16(13):2336-2339. doi: 10.1021/acschemneuro.5c00383. Epub 2025 Jun 17. ACS Chem Neurosci. 2025. PMID: 40527057 Review.
References
-
- Suthar P., Kumar S., Kumar V., Vaidya D., Sharma A., Sharma A. Murraya koenigii (L.) Spreng: Speculative ethnobotanical perspectives of ubiquitous herb with versatile nutra/functional properties. South Afr. J. Bot. 2022;145:111–134. doi: 10.1016/j.sajb.2021.11.025. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

