Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle-Cinnamic Acid Hybrids
- PMID: 40807323
- PMCID: PMC12348798
- DOI: 10.3390/molecules30153148
Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle-Cinnamic Acid Hybrids
Abstract
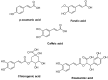
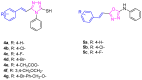
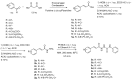
In this study, the design and synthesis of a novel series of cinnamic acid and 1,2,4-triazole hybrids were reported, aiming to enhance antioxidant and lipoxygenase inhibitory activities through pharmacophore combination. Cinnamic acid derivatives and 1,2,4-triazoles exhibit a broad spectrum of biological activities; therefore, by synthesizing hybrid molecules, we would like to exploit the beneficial characteristics of each scaffold. The general synthetic procedure comprises three synthetic steps, starting from the reaction of appropriate substituted cinnamic acid with hydrazine monohydrate in acetonitrile with cyclohexane and resulting in the formation of hydrazides. Consequently, the hydrazides reacted with phenylisothiocyanate under microwave irradiation conditions. Then, cyclization proceeded to the 1,2,4-triazole after the addition of NaOH solution and microwave irradiation. All the synthesized derivatives have been studied for their ability (a) to interact with the free radical DPPH, (b) inhibit lipid peroxidation induced by AAPH, and (c) inhibit soybean lipoxygenase. The synthesized derivatives have shown significant antioxidant activity and have been proved to be very good lipoxygenase inhibitors. Compounds 4b and 4g (IC50 = 4.5 μM) are the most potent within the series followed by compound 6a (IC50 = 5.0 μM). All the synthesized derivatives have been subjected to docking studies related to soybean lipoxygenase. Compound 4g exhibited a docking score of -9.2 kcal/mol and formed hydrophobic interactions with Val126, Tyr525, Lys526, Arg533, and Trp772, as well as a π-cation interaction with Lys526.
Keywords: 1,2,4-triazole; 1,3,4-oxadiazole; anti-inflammatory; antioxidant; chimeric molecules; cinnamic acid derivatives.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





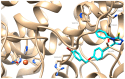

References
-
- El-Serwy W.S., Mohamed N.A., Abbas E.M., Abdel-Rahman R.F. Synthesis and anti-inflammatory properties of novel 1,2,4-triazole derivatives. Res. Chem. Intermed. 2013;39:2543–2554. doi: 10.1007/s11164-012-0781-9. - DOI
-
- Matore B.W., Banjare P., Guria T., Roy P.P., Singh J. Oxadiazole derivatives: Histone deacetylase inhibitors in anticancer therapy and drug discovery. Eur. J. Med. Chem. Rep. 2022;5:100058. doi: 10.1016/j.ejmcr.2022.100058. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

