Cathodic Exfoliation of Various Graphite Materials in Potassium Chloride Electrolyte
- PMID: 40807326
- PMCID: PMC12348432
- DOI: 10.3390/molecules30153151
Cathodic Exfoliation of Various Graphite Materials in Potassium Chloride Electrolyte
Abstract
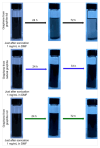
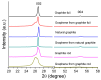
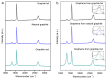
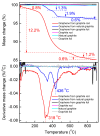
Cathodic exfoliation of graphite has emerged as an attractive method to synthesize high-quality and lo- defect graphene. Here, it is demonstrated that the type of starting graphite material influences the properties of exfoliated graphene. Graphite foil, natural graphite, and graphite rods were examined in the exfoliation processes performed in 3.0 M KCl at -15 V. The use of a graphite foil facilitates the rapid cathodic exfoliation process in comparison with structurally more compact natural graphite and graphite rods. For the graphite foil, the cathodically exfoliated graphene exhibits a low defect density (ID/IG of 0.09, a C/O ratio of 35) with graphite exfoliation yield of 92.8%. In contrast, the exfoliated graphene from natural graphite exhibits an ID/IG of 0.15, a C/O ratio of 28, and a graphite exfoliation yield of 30.5%, whereas graphene from graphite rod exhibits an ID/IG of 0.86, a C/O ratio of 30, and a graphite exfoliation yield of 19.5%. The dense structure of natural graphite and graphite rods led to longer exfoliation times. Exfoliation of graphite rods produced few-layer graphene with the smallest sheet size, whereas natural graphite and graphite foil yielded multilayer graphene with larger sheets. This study demonstrates the feasibility of using aqueous-based cathodic exfoliation to produce graphene from various graphite sources, leading to variations in sheet thickness, size, defect density, and solvent dispersibility.
Keywords: cathodic exfoliation; degree of graphitization; graphene; graphite sources.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Low C.T.J., Walsh F.C., Chakrabarti M.H., Hashim M.A., Hussain M.A. Electrochemical Approaches to the Production of Graphene Flakes and their Potential Applications. Carbon. 2013;54:1–21. doi: 10.1016/j.carbon.2012.11.030. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

