Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson's Disease
- PMID: 40807329
- PMCID: PMC12348509
- DOI: 10.3390/molecules30153154
Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson's Disease
Abstract
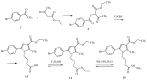
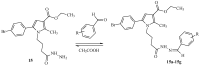
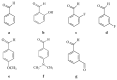
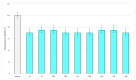
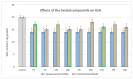
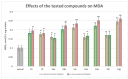
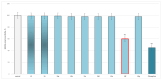
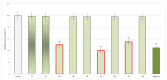
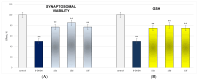
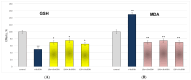
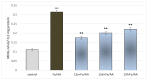
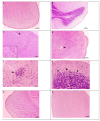
Some studies performed in our laboratory on pyrrole and its derivatives pointed towards the enrichment of the evaluations of these promising chemical structures for the potential treatment of neurodegenerative conditions in general and Parkinson's disease in particular. A classical Paal-Knorr cyclization approach is applied to synthesize the basic hydrazine used for the formation of the designed series of hydrazones (15a-15g). The potential neurotoxic and neuroprotective effects of the newly synthesized derivatives were investigated in vitro using different models of induced oxidative stress at three subcellular levels (rat brain synaptosomes, mitochondria, and microsomes). The results identified as the least neurotoxic molecules, 15a, 15d, and 15f applied at a concentration of 100 µM to the isolated fractions. In addition, the highest statistically significant neuroprotection was observed for 15a and 15d at a concentration of 100 µM using three different injury models on subcellular fractions, including 6-hydroxydopamine in rat brain synaptosomes, tert-butyl hydroperoxide in brain mitochondria, and non-enzyme-induced lipid peroxidation in brain microsomes. The hMAOA/MAOB inhibitory activity of the new compounds was studied at a concentration of 1 µM. The lack of a statistically significant hMAOA inhibitory effect was observed for all tested compounds, except for 15f, which showed 40% inhibitory activity. The most prominent statistically significant hMAOB inhibitory effect was determined for 15a, 15d, and 15f, comparable to that of selegiline. The corresponding selectivity index defined 15f as a non-selective MAO inhibitor and all other new hydrazones as selective hMAOB inhibitors, with 15d indicating the highest selectivity index of >471. The most active and least toxic representative (15d) was evaluated in vivo on Rotenone based model of Parkinson's disease. The results revealed no microscopically visible alterations in the ganglion and glial cells in the animals treated with rotenone in combination with 15d.
Keywords: Parkinson’s; hydrazone; in vivo; pyrrole; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Zafar S., Yaddanapudi S.S. StatPearls. StatPearls Publishing; Tampa, FL, USA: 2023. Parkinson Disease. - PubMed
-
- Sensi S.L., Russo M., Tiraboschi P. Biomarkers of diagnosis, prognosis, pathogenesis, response to therapy: Convergence or divergence? Lessons from Alzheimer’s disease and synucleinopathies. Handb. Clin. Neurol. 2023;192:187–218. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

