Straightforward Access to the Dispirocyclic Framework via Regioselective Intramolecular Michael Addition
- PMID: 40807339
- PMCID: PMC12348457
- DOI: 10.3390/molecules30153164
Straightforward Access to the Dispirocyclic Framework via Regioselective Intramolecular Michael Addition
Abstract
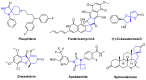
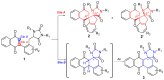
In this article, an efficient and straightforward protocol for the construction of complex dispirocyclic skeletons via regioselective intramolecular Michael addition is presented. Diverse dispirocyclic compounds were synthesized under mild and transition-metal-free conditions with good to excellent yields. Most stereoisomers were conveniently separated by column chromatography, and their relative configurations were identified by single-crystal X-Ray diffraction of representative compounds. A scale-up experiment validated the practicality of this method. In an in vitro assay, some dispirocyclic compounds exhibited potent cytotoxicity with an IC50 value of 10-6 mol/L.
Keywords: regioselectivity; spiro compounds; transition-metal free.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Digital interventions in mental health: evidence syntheses and economic modelling.Health Technol Assess. 2022 Jan;26(1):1-182. doi: 10.3310/RCTI6942. Health Technol Assess. 2022. PMID: 35048909 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Patil S.P., Pacitti M.F., Gilroy K.S., Ruggiero J.C., Griffin J.D., Butera J.J., Notarfrancesco J.M., Tran S., Stoddart J.W. Identification of antipsychotic drug fluspirilene as a potential p53-MDM2 inhibitor: A combined computational and experimental study. J. Comput. Aided Mol. Des. 2015;29:155–163. doi: 10.1007/s10822-014-9811-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

