Efficient Tetracycline Hydrochloride Degradation by Urchin-Like Structured MoS2@CoFe2O4 Derived from Steel Pickling Sludge via Peroxymonosulfate Activation
- PMID: 40807378
- PMCID: PMC12348658
- DOI: 10.3390/molecules30153194
Efficient Tetracycline Hydrochloride Degradation by Urchin-Like Structured MoS2@CoFe2O4 Derived from Steel Pickling Sludge via Peroxymonosulfate Activation
Abstract
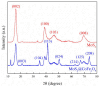
Steel pickling sludge serves as a valuable iron source for synthesizing Fe-based catalysts in heterogeneous advanced oxidation processes (AOPs). Here, MoS2@CoFe2O4 catalyst derived from steel pickling sludge was prepared via a facile solvothermal approach and utilized to activate peroxymonosulfate (PMS) for tetracycline hydrochloride (TCH) degradation. Comprehensive characterization using scanning electron microscopy (SEM)-energy dispersive spectrometer (EDS), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) confirmed the supported microstructure, composition, and crystalline structure of the catalyst. Key operational parameters-including catalyst dosage, PMS concentration, and initial solution pH-were systematically optimized, achieving 81% degradation efficiency within 30 min. Quenching experiments and electron paramagnetic resonance (EPR) analysis revealed SO4∙- as the primary oxidative species, while the catalyst maintained high stability and reusability across cycles. TCH degradation primarily occurs through hydroxylation, decarbonylation, ring-opening, and oxidation reactions. This study presents a cost-effective strategy for transforming steel pickling sludge into a high-performance Fe-based catalyst, demonstrating its potential for practical AOP applications.
Keywords: MoS2@CoFe2O4 catalyst; degradation; peroxymonosulfate; pickling sludge; tetracycline hydrochloride.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Dias I.M., Mourão L.C., Andrade L.A., Souza G.B.M., Viana J.C.V., Oliveira S.B., Alonso C.G. Degradation of antibiotic amoxicillin from pharmaceutical industry wastewater into a continuous flow reactor using supercritical water gasification. Water Res. 2023;234:119826. doi: 10.1016/j.watres.2023.119826. - DOI - PubMed
-
- Li S., Zhang T., Zheng H., Dong X., Leong Y.K., Chang J.S. Advances and challenges in the removal of organic pollutants via sulfate radical-based advanced oxidation processes by Fe-based metal-organic frameworks: A review. Sci. Total Environ. 2024;926:171885. doi: 10.1016/j.scitotenv.2024.171885. - DOI - PubMed
-
- Zhang X., Wei J., Wang C., Wang L., Guo Z., Song Y. Recent advance of Fe-based bimetallic persulfate activation catalysts for antibiotics removal: Performance, mechanism, contribution of the key ROSs and degradation pathways. Chem. Eng. J. 2024;487:150514. doi: 10.1016/j.cej.2024.150514. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

