Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds-Part 1: Aromatic Frameworks
- PMID: 40807439
- PMCID: PMC12348598
- DOI: 10.3390/molecules30153264
Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds-Part 1: Aromatic Frameworks
Abstract
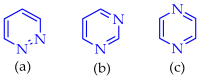
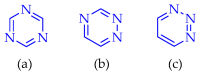
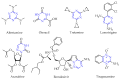
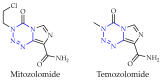
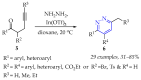
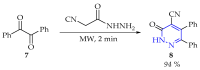
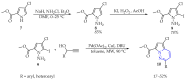
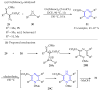
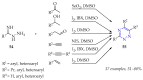
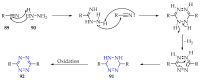
This review summarises the possible applications and basic methodologies for the synthesis of six-membered polyazo heterocycles, namely, diazines, triazines, and tetrazines. The time period covered by the analysed works ranges from the beginning of the 20th century to the present day. This period was chosen because it was during this time that synthetic chemistry, as defined by physicochemical research methods, became capable of solving such complex problems as efficiently as possible. The first part of the review describes the applications of polyazo heterocyclic compounds, whose frameworks are found in the composition of drugs, dyes, and functional molecules for materials chemistry, as well as in a wide variety of natural compounds and their synthetic analogues. The review also systematises the methods for assembling six-membered aromatic polyazo heterocycles, including intramolecular and sequential cyclisation, which determine the possible structural and functional diversity based on the presence and arrangement of nitrogen atoms and the position of the corresponding substituents.
Keywords: azaheterocyclic; diazine; tetrazine; triazine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

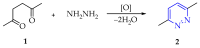
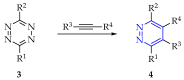

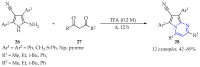
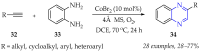
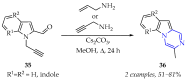
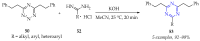
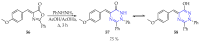
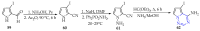


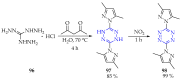
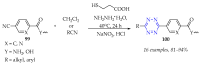
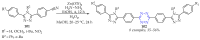
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Unveiling the Therapeutic Potential of Compounds Carrying Mono- and Bis-Thiazole Nuclei with Distinct Heterocyclic Analogues for Addressing Cancer Challenges.Curr Cancer Drug Targets. 2025 Jun 20. doi: 10.2174/0115680096375796250610065705. Online ahead of print. Curr Cancer Drug Targets. 2025. PMID: 40574393
References
-
- Abida, Alam M.T., Asif M. Pharmacological activities of pyridoxines and pyridazinone Derivatives: A Review on biologically active scaffold. SAR J. Pharm. Sci. 2019;1:16–37. doi: 10.36346/sarjps.2019.v01i01.004. - DOI
-
- Asif M., Alghamdi S. A mini-review on pyridazine analogs: Chemical and pharmacological potentials. Mini Rev. Org. Chem. 2023;20:100–123. doi: 10.2174/1570193X19666220329155551. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

