Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET-Derived Waste Treatment
- PMID: 40807477
- PMCID: PMC12348328
- DOI: 10.3390/molecules30153302
Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET-Derived Waste Treatment
Abstract
Polyethylene terephthalate (PET) is a widely used polymer whose accumulation in the environment poses a significant pollution challenge. This study explores the degradation of bis(2-hydroxyethyl) terephthalate (BHET) and terephthalic acid (TPA)-two monomers commonly produced during PET hydrolysis and widely used as intermediates in PET recycling-through Advanced Oxidation Processes (AOPs) employing KrCl (222 nm) and XeBr (283 nm) excimer lamps in the presence of hydrogen peroxide (H2O2). The effects of the H2O2/monomer mass ratio, initial monomer concentrations, and reaction volume on degradation efficiency were systematically evaluated. The results demonstrate that excimer lamp technology, particularly KrCl, holds promising potential for the effective degradation of both BHET and TPA, and thus represents a viable strategy for PET waste treatment.
Keywords: advanced oxidation; bis(2-hydroxyethyl) terephthalate; excimer radiation; microplastic; polyethylene terephthalate; terephthalic acid.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
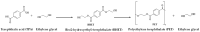











References
-
- CSIC Combatir La Contaminación Por Plásticos. 2023. [(accessed on 4 May 2025)]. Available online: https://www.idaea.csic.es/wp-content/uploads/2023/11/Ciencia-para-las-po....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

