Synthesis and Biological Evaluation of β-Phenylalanine Derivatives Containing Sulphonamide and Azole Moieties as Antiproliferative Candidates in Lung Cancer Models
- PMID: 40807478
- PMCID: PMC12348707
- DOI: 10.3390/molecules30153303
Synthesis and Biological Evaluation of β-Phenylalanine Derivatives Containing Sulphonamide and Azole Moieties as Antiproliferative Candidates in Lung Cancer Models
Abstract
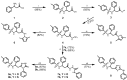
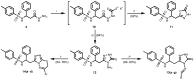
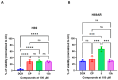
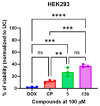
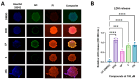
In this study, a series of novel β-phenylalanine derivatives were synthesised and evaluated for their anticancer activity. The 3-(4-methylbenzene-1-sulfonamido)-3-phenylpropanoic acid (2) was prepared using β-phenylalanine as a core scaffold. The β-amino acid derivative 2 was converted to the corresponding hydrazide 4, which enabled the development of structurally diverse heterocyclic derivatives including pyrrole 5, pyrazole 6, thiadiazole 8, oxadiazole 11, triazoles 9 and 12 with Schiff base analogues 13 and series1,2,4-triazolo [3,4-b][1,3,4]thiadiazines 14. These modifications were designed to enhance chemical stability, solubility, and biological activity. All compounds were initially screened for cytotoxicity against the A549 human lung adenocarcinoma cell line, identifying N-[3-(3,5-dimethyl-1H-pyrazol-1-yl)-3-oxo-1-phenylpropyl]-4-methylbenzenesulfonamide (5) and (E)-N-{2-[4-[(4-chlorobenzylidene)amino]-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl]-1-phenylethyl}-4-methylbenzenesulfonamide (13b) as the most active. The two lead candidates were further evaluated in H69 and H69AR small cell lung cancer lines to assess activity in drug-sensitive and multidrug-resistant models. Schiff base 13b containing a 4-chlorophenyl moiety, retained potent antiproliferative activity in both H69 and H69AR cells, comparable to cisplatin, while compound 5 lost efficacy in the resistant phenotype. These findings suggest Schiff base derivative 13b may overcome drug resistance mechanisms, a limitation commonly encountered with standard chemotherapeutics such as doxorubicin. These results demonstrate the potential role of β-phenylalanine derivatives, azole-containing sulphonamides, as promising scaffolds for the development of novel anticancer agents, particularly in the context of lung cancer and drug-resistant tumours.
Keywords: 1,2,4-triazolo [3,4-b][1,3,4]thiadiazine; H69AR cells; anticancer agents; azole; oxadiazole; sulphanilamide; triazole; β-phenylalanine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
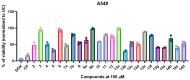
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Epidemiology of Triazole Resistant Aspergillus fumigatus in Asia: A Systematic Review and Meta-Analysis.Mycoses. 2025 Aug;68(8):e70099. doi: 10.1111/myc.70099. Mycoses. 2025. PMID: 40792442 Review.
-
Synthesis and multi-target antiproliferative evaluation of novel 1,2,4-triazole-3-thione analogues against breast cancer: in silico and in vitro mechanistic insights.RSC Adv. 2025 Jul 14;15(30):24769-24790. doi: 10.1039/d5ra02512e. eCollection 2025 Jul 10. RSC Adv. 2025. PMID: 40661216 Free PMC article.
-
Efficient Synthesis, Anticancer Evaluation of Triazole-Thiadiazole/Benzo[d]Oxazole Scaffolds, and Investigation of Their Reactivity Properties Using Density-Functional Theory Calculations and In Silico Docking.Chem Biodivers. 2025 Jul;22(7):e202402470. doi: 10.1002/cbdv.202402470. Epub 2025 Mar 11. Chem Biodivers. 2025. PMID: 39972950
References
-
- Zhu Q., Tao Y., Han Y., He Y., Fu Y., Yang H., Chen Y., Shi Y. Quercetin Alleviates Breast Cancer-Related Depression by Inhibiting Neutrophil Extracellular Traps via Inhibition of Sphingosine 1-Phosphate/Sphingosine 1-Phosphate Receptor Axis. Phytother. Res. 2025;39:2848–2862. doi: 10.1002/ptr.8513. - DOI - PubMed
-
- Laroche F.J.F., Li S., Shen N., Hwang S.K., Nguyen G., Yu W., Wong C.K., Quinton R.J., Berman J.N., Liu C.T., et al. S1P1 Threonine 236 Phosphorylation Mediates the Invasiveness of Triple-Negative Breast Cancer and Sensitivity to FTY720. Cells. 2023;12:980. doi: 10.3390/cells12070980. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

