GelMA Core-Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing
- PMID: 40807480
- PMCID: PMC12348063
- DOI: 10.3390/molecules30153305
GelMA Core-Shell Microgel Preparation Based on a Droplet Microfluidic Device for Three-Dimensional Tumor Ball Culture and Its Drug Testing
Abstract
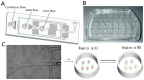
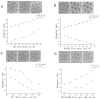
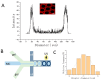
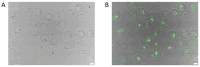
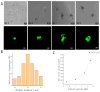
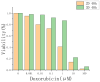
Gelatin methacrylate (GelMA) microgels serve as promising bioscaffolds for tissue engineering and drug screening. However, conventional solid GelMA microgels often exhibit limited mass transfer efficiency and provide insufficient protection for embedded cells. In this study, we developed a droplet-based microfluidic platform to fabricate core-shell structured GelMA microgels. This system enabled precise control over microgel size and core-to-shell ratio by modulating flow rates. Encapsulation of A549 cells within these core-shell microgels preserved cellular viability and facilitated the formation of three-dimensional tumor spheroids. These outcomes confirmed both the protective function of the core-shell architecture during encapsulation and the overall biocompatibility of the microgels. The developed GelMA core-shell microgel system presents considerable applicability in research domains such as organoid modeling and high-throughput pharmacological screening.
Keywords: 3D cell culture; GelMA; core–shell microgel; droplet microfluidic; drug testing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
On-chip fabrication and in-flow 3D-printing of microgel constructs: from chip to scaffold materials in one integral process.Biofabrication. 2024 Mar 28;16(2). doi: 10.1088/1758-5090/ad3318. Biofabrication. 2024. PMID: 38471160
-
Microfluidic-based gelatin methacrylate microgel as a scaffold to create reverse-polarity HT29 spheroids.Int J Biol Macromol. 2025 May;305(Pt 1):140824. doi: 10.1016/j.ijbiomac.2025.140824. Epub 2025 Feb 13. Int J Biol Macromol. 2025. PMID: 39954894
-
Printing GelMA bioinks: a strategy for buildingin vitromodel to study nanoparticle-based minocycline release and cellular protection under oxidative stress.Biofabrication. 2024 Mar 28;16(2). doi: 10.1088/1758-5090/ad30c3. Biofabrication. 2024. PMID: 38447206
-
Research progress on stiffness controllable scaffolds based on gelatin methacryloyl hydrogels for tissue repair and reconstruction.Int J Biol Macromol. 2025 Sep;321(Pt 3):146485. doi: 10.1016/j.ijbiomac.2025.146485. Epub 2025 Jul 31. Int J Biol Macromol. 2025. PMID: 40752703 Review.
-
Innovations in cancer treatment: evaluating drug resistance with lab-on-a-chip technologies.Int J Pharm. 2025 Sep 15;682:125936. doi: 10.1016/j.ijpharm.2025.125936. Epub 2025 Jul 5. Int J Pharm. 2025. PMID: 40623610 Review.
References
-
- Augustine R., Zahid A.A., Hasan A., Dalvi Y.B., Jacob J. Cerium oxide nanoparticle-loaded gelatin methacryloyl hydrogel wound-healing patch with free radical scavenging activity. ACS Biomater. Sci. Eng. 2021;7:279–290. - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFC3502005/National Key Research and Development Program of China
- 2022YFC3502002/National Key Research and Development Program of China
- 2022YFC3500115/National Key Research and Development Program of China
- 101GJHZ2024017M1/CAS-NSTDA Joint Research Project, China-Thailand (2024)
- 20XD1404600/Program of Shanghai Academic/Technology Research Leader
LinkOut - more resources
Full Text Sources

